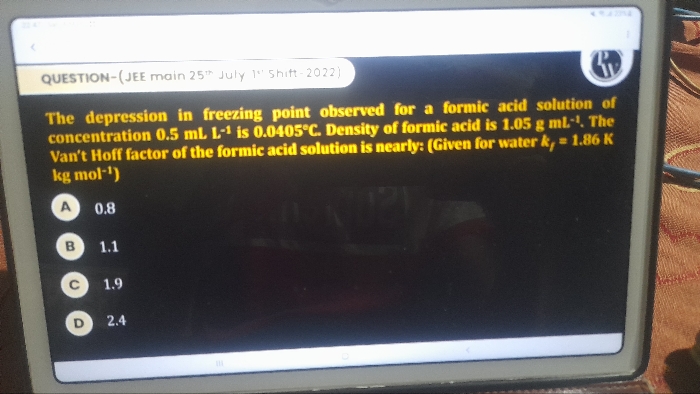
JEE Class main Answered
Calculate the molar conductance of 0.01M solution of an electrolyte which has a resistance of 210ohms at 298K. Cell constant is 0.88cm-1
Asked by mrudulmahadev1311 | 20 Aug, 2019, 20:40: PM
Given:
Resistance = 210 Ω
Concentration of solution = 0.1 M
Cell constant = 0.88 cm−1
Specific conductance

Molar conductance

Answered by Varsha | 21 Aug, 2019, 11:04: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM
JEE main - Chemistry
Asked by debnath.ankita2023 | 16 May, 2024, 13:35: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 20:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 14:23: PM