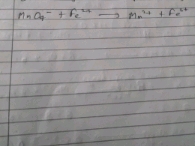CBSE Class 11-science Answered
calculate the mass of potassium chlorate required to liberate 6.72 dm3 of oxygen at STP , molar mass of KCl3 is 112,5 g/mol.
Asked by | 24 May, 2012, 03:45: PM
1 dm3 = 1 L
2KClO3 ? 2KCl + 3O2
From equation ,
22.4 x 3 dm3 liberate oxygen by = 2 x 112.5 g KClO3
1 liberate oxygen by = 112.5 x2 / 3 x 22.4
6.72 dm3 ' liberate oxygen by '= 6.72 x 112.5 x 2 / 3 x 22.4
= 225 g
Answered by | 31 May, 2012, 10:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM