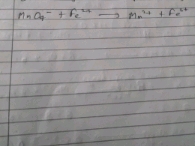CBSE Class 11-science Answered
calculate the mass of Mg.................
Asked by | 21 Aug, 2008, 11:52: PM
23 g of Na contains = 6.023 x 1023atoms
1gof Na contains = 6.023 x 1023/ 23atoms
2.3gof Na contains = 6.023 x 1023/ 23atoms X 2.3
=6.023x 1022atoms = Mg atoms
so, if 6.023x 1023atoms have =24 g
in 6.023x 1022atoms mass = 24 x 6.023x 1022atoms / 6.023 x 1023
= 2.4g
Answered by | 22 Aug, 2008, 02:45: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM