CBSE Class 10 Answered
Assertion- Silver articles become coated with a black layer upon exposure to air.
Resaon- A layer of oxide gets deposited upon oxidation with air.
A- Assertion and Reason both are correct and reason is the correct explanation of assertion.
B- Assertion and Reason both are correct and reason is not the correct explanation of assertion.
C- Assertion is true but reason is false.
D- Reason is true but assertion is false
Please provide an explanation too.
Asked by vandanawasan02 | 13th Feb, 2020, 09:24: AM
Expert Answer:
Aseertion and reason both are correct and reason is correct explanation of assertion. When silver is exposed to air, it becomes black due to oxide coating, although silver is not very reactive metal but with passage of time, it slowly reacts with oxygen in the air and forms black coloures silver oxide.
Follow up question- Silver forms sulphide layer upon oxidation. I am confused between B and C. A option is definitely wrong.
Asked by vandanawasan02 | 13 Feb, 2020, 02:08: PM
Silver also forms Silver Sulphide because of reaction in between traces of H2S present in environment. Silver reacts with oxygen and forms Silver Oxide. Silver Sulphide is formed in more quantity as compared to Silver oxide because Silver has more affinity to form a bond with S as compared to O. Ag reacts very slow with O.
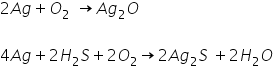
So Oxide is deposited but colour of Ag2O is brown in layer and colour of Ag2S is black.
So Silver is oxidised and forms Silver sulphide and Silver oxide. But black colour of layer is formed due to sulphide formation (due to high affinity with silver) which forms when silver is exposed to air.
Answered by Ravi | 13 Feb, 2020, 03:18: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by ten.foundation | 29 Aug, 2022, 07:53: PM
CBSE 10 - Chemistry
Asked by bhuyanagas | 30 Nov, 2021, 09:46: PM
CBSE 10 - Chemistry
Asked by s158410a.niraj007031 | 03 Nov, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by shlok4559 | 01 Nov, 2021, 09:39: PM
CBSE 10 - Chemistry
Asked by pardeep360sehlang | 06 Oct, 2021, 09:28: PM
CBSE 10 - Chemistry
Asked by yogipharma11 | 22 Oct, 2020, 06:28: PM
CBSE 10 - Chemistry
Asked by nehchalrs15 | 02 Oct, 2020, 10:14: AM
CBSE 10 - Chemistry
Asked by nirupathak2004 | 21 May, 2020, 06:09: PM
CBSE 10 - Chemistry
Asked by bhuvaneswari5781 | 18 May, 2020, 11:13: AM
CBSE 10 - Chemistry
Asked by debjit_dm | 01 May, 2020, 04:41: PM









