CBSE Class 10 Answered
How to find redox reaction?
Asked by bhuvaneswari5781 | 18 May, 2020, 11:13: AM
A chemical reaction in which loss of electrons and gain of electrons occur simultaneously is called a redox reaction.
Example:
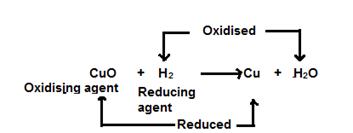
In this reaction, hydrogen acts as a reducing agent and reduces copper oxide to copper. This is a reduction reaction.
Reduction: Cu+2 + 2e−→ Cu
Simultaneously, copper oxide acts as an oxidising agent and oxidises hydrogen to water. This is an oxidation reaction.
Oxidation: 2H - 2e− → 2H+
There is one more way a reaction called redox is as follows:
Redox reactions: Redox reactions are those reactions in which both oxidation and reduction occur.
Oxidation - involves the loss of hydrogen OR gain of oxygen
Reduction - involves the gain of hydrogen OR loss of oxygen
ZnO + C → Zn +CO
In this reaction, zinc oxide is reduced to zinc and Carbon is oxidized to carbon monoxide
Oxidation - involves the loss of hydrogen OR gain of oxygen
Reduction - involves the gain of hydrogen OR loss of oxygen
ZnO + C → Zn +CO
In this reaction, zinc oxide is reduced to zinc and Carbon is oxidized to carbon monoxide
Answered by Ramandeep | 18 May, 2020, 11:18: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by ten.foundation | 29 Aug, 2022, 07:53: PM
CBSE 10 - Chemistry
Asked by bhuyanagas | 30 Nov, 2021, 09:46: PM
CBSE 10 - Chemistry
Asked by s158410a.niraj007031 | 03 Nov, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by shlok4559 | 01 Nov, 2021, 09:39: PM
CBSE 10 - Chemistry
Asked by pardeep360sehlang | 06 Oct, 2021, 09:28: PM
CBSE 10 - Chemistry
Asked by yogipharma11 | 22 Oct, 2020, 06:28: PM
CBSE 10 - Chemistry
Asked by nehchalrs15 | 02 Oct, 2020, 10:14: AM
CBSE 10 - Chemistry
Asked by nirupathak2004 | 21 May, 2020, 06:09: PM
CBSE 10 - Chemistry
Asked by bhuvaneswari5781 | 18 May, 2020, 11:13: AM
CBSE 10 - Chemistry
Asked by debjit_dm | 01 May, 2020, 04:41: PM









