NEET Class neet Answered
A solution M is prepared by mixing ethanol and H2O.The mole fraction of ethanol in mixture is 0.9 . Given: Kf H20-1.86 Kkg/mol ,Kf ethanol-2.0 Kkg/mol ,Kb H2O-0.52 Kkg/mol ,Kb ethanol-1.2 Kkg/mol, Standard freezing point of H20- 273K , Standard freezing point of ethanol- 155.7K , Standard boiling point of H2O- 373K , Standard boiling point of ethanol-351.5K ,vp of pure water -32.8mm Hg,vp of pure ethanol -40mm Hg, mol wt of H2O - 18g/mol, mol wt of ethanol- 46g/mol .
Consider the solutions to be ideal dilute solutions and solutes to be non volatile and non-dissociative .Ques 1 ) The fp of solution M is: (a) 268.7K ,(b) 268.5 K ,(c) 234.2 K , (d) 150.9 K .Ques 2 ) The vp of solution M is: (a) 39.3mmHg ,(b) 36.0mmHg ,(c) 39.5mmHg ,(d) 28.8mmHg .Ques 3) Wter is added to the solution M such that the mole fraction of water in solution becomes 0.9. The bp of this solution is : (a)380.4K ,(b)376.2K, (c)375.5K ,(d)354.7K
Asked by Balbir | 30 Jul, 2019, 18:16: PM
Given:
Mole fraction of ethanol = 0.9
Mole fraction of water = 1-0.9
= 0.1
Kf H2O = 1.86 K kg/mol
Kf ethanol = 2.0 K kg/mol
Kb H2O = 0.52 K kg/mol
Kb ethanol = 1.2 K kg/mol
Standard freezing point of H20 =273 K
Standard freezing point of ethanol = 155.7 K
Standard boiling point of H2O = 373 K
Standard boiling point of ethanol=351.5 K
Vapour pressure of pure water = 32.8 mm Hg
Vapour pressure of pure ethanol = 40 mm Hg
Molecular weight of H2O - 18 g/mol
Molecular weight of ethanol = 46 g/mol
1) Freezing point of solution M,
We know,

Freezing point of solution = 155.7 - 4.83
=150.86 K
2) Vapour pressure of solution M is:
P = P1X1 + P2X2
= 32.8 × 0.1 + 40 × 0.9
=3.28 +36
= 39.28 mm Hg
≈ 39.3 mm Hg
3) Water is added to the solution M such that the mole fraction of water in solution becomes 0.9.
The bp of this solution is,
We know,
Elevation in boiling point is
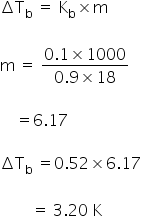
Boiling point of solution = 373 + 3.20
=376.20 K
Answered by Varsha | 31 Jul, 2019, 12:47: PM
Concept Videos
NEET neet - Chemistry
Asked by hdjsiisisii | 03 Aug, 2024, 06:21: AM
NEET neet - Chemistry
Asked by kanishksingh538 | 22 May, 2024, 00:27: AM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 13:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 14:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 21:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 22:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 13:42: PM