CBSE Class 10 Answered
A non-metal A which is the largest constituent of air combines with hydrogen on heating in the presence of Fe (as catalyst) and forms a gas B. When this gas B is treated with H²SO4, compound C is formed which is broadly used as chemical fertiliser.
(¡) identify A, b and C.
(¡¡)locate the position of A in the mordern periodic table.
(¡¡¡)which elements are present before and after the element A ?
(¡v) write the electronic configuration of element A.
Asked by pdcavita | 29 Nov, 2017, 10:09: PM
Non-metal A is Nitrogen which is the largest constituent of air combines with hydrogen on heating in the presence of Fe (as catalyst) and forms a gas B i.e., Ammonia NH3
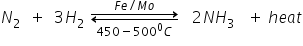
When this ammonia gas is treated with H2SO4, compound C i.e. ammonium sulphate is formed which is broadly used as chemical fertiliser.
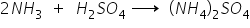
i) Compound A is Nitrogen Compound B is Ammonia and compound C is Ammonium nitrate.
ii) Position of Nitrogen in periodic table = Group is 15, VA and period is 2
iii) Carbon is present before the Nitrogen and Oxygen is present after the oxygen.
iv) electronic configuration of nitrogen : 1s2 2s2 2p3
Answered by Ramandeep | 30 Nov, 2017, 12:55: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by shettyshrinidhi271 | 07 Jan, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by adipadmakarri | 05 Dec, 2020, 06:41: PM










