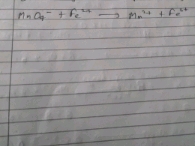CBSE Class 11-science Answered
A granulated sample of aircraft alloy(Al,Mg,Cu) weighing 8.72 g was first treated with alkali to dissolved Al, then with very dilute HCl to dissolve Mg,leaving a residue of Cu. The residue after alkali boiling weighed 2.1 g and the acid insoluble residue from this weighed 0.69g. What is the composition of the alloy?
Asked by Prince Sonu | 18 Apr, 2013, 09:29: PM
We know that the amount lost during alkali boiling is the total amount of aluminum: subtract 2.1g from 8.72g to get 6.62g Al. The residue after acid is the mass of the copper: .69g Cu. The mass of Mg is the amount that dissolved in the acid: 2.1g - .69g = 1.41g.
Divide each mass by the total amount of alloy used and multiply by 100% to get the percentage of each element in the alloy.
(6.62/8.72) x 100% = 75.9% Al
(1.14/8.72) x 100% = 13.1% Mg
(0.69/8.72) x 100% = 7.9% Cu
Divide each mass by the total amount of alloy used and multiply by 100% to get the percentage of each element in the alloy.
(6.62/8.72) x 100% = 75.9% Al
(1.14/8.72) x 100% = 13.1% Mg
(0.69/8.72) x 100% = 7.9% Cu
Answered by | 19 Apr, 2013, 08:59: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM