CBSE Class 11-science Answered
A gaseous mixture contains CO and SO2 in equimolecular proportion.The weight of 2.24 L of this mixture at NTP is?
Asked by pb_ckt | 19 May, 2019, 23:54: PM
Suppose we are taking one miole of CO and SO2 .
1 mole of Co weight is =28 g
1 mole of So2 weight is=64 g
Volume of 1 mole of CO is=22.4 L
Volume of 1 mole of SO2 is=22.4 L
So Toatal volume=22.4+22.4=44.8 L
44.8 L of volume will have total weight=64+28=92g
So 1 L will have=92/44.8
2.24 will have weight=
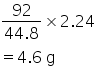
Answered by Ravi | 20 May, 2019, 15:56: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mohd.arhaan812 | 06 May, 2024, 21:55: PM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 22:43: PM
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 20:58: PM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 00:09: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 14:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 22:07: PM
CBSE 11-science - Chemistry
Asked by devanshuchhipani | 12 Mar, 2020, 20:42: PM
CBSE 11-science - Chemistry
Asked by ritua7330 | 01 Sep, 2019, 12:53: PM
CBSE 11-science - Chemistry
Asked by sajidivakaran | 21 Jul, 2019, 09:41: AM
CBSE 11-science - Chemistry
Asked by shashank1854 | 28 May, 2019, 16:48: PM