CBSE Class 12-science Answered
A and B have identical size and masses. A becomes A++ and B becomes B--.. Will A++ and B-- will have the same mass...???
Asked by Mohit Panchal | 26 Apr, 2011, 04:26: AM
aWe know that atomic mass of an atom is the total sum of neutrons, protons and electrons. However, electrons contribute less than 0.06% to an atom's total mass.
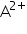 means atom A has lost 2 electrons from it so the mass of it is reduced by mass of two electrons.
means atom A has lost 2 electrons from it so the mass of it is reduced by mass of two electrons.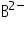 means atom B has gain 2 electrons, so its mass will be increased by mass of two electrons.
means atom B has gain 2 electrons, so its mass will be increased by mass of two electrons.In this case you can give two answer as mass of the electrons is almost negligible to the mass of an atom, so we can say that atom 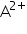 and
and 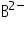 have the same mass. But if we dint neglect the mass of the electron then we can say that atom
have the same mass. But if we dint neglect the mass of the electron then we can say that atom 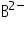 is bigger in mass then atom
is bigger in mass then atom 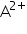 .
.
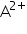 and
and 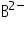 have the same mass. But if we dint neglect the mass of the electron then we can say that atom
have the same mass. But if we dint neglect the mass of the electron then we can say that atom 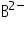 is bigger in mass then atom
is bigger in mass then atom 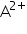 .
.Mass of a electron is 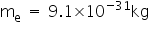
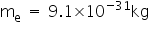
Mass of a proton is 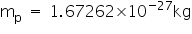
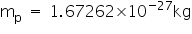
Mass of a neutron is 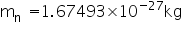
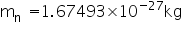
Answered by | 27 Apr, 2011, 01:04: AM
Concept Videos
CBSE 12-science - Physics
Asked by niharvijayvargiya5 | 23 Apr, 2024, 06:40: PM
CBSE 12-science - Physics
Asked by adityagalar2007 | 06 Apr, 2024, 01:06: PM
CBSE 12-science - Physics
Asked by amlanmcob | 06 Apr, 2024, 12:27: PM
CBSE 12-science - Physics
Asked by hussain221man | 05 Apr, 2024, 08:44: PM
CBSE 12-science - Physics
Asked by manishamunda787 | 02 Apr, 2024, 11:07: AM
CBSE 12-science - Physics
Asked by am1954077 | 08 Mar, 2024, 04:57: PM
CBSE 12-science - Physics
Asked by rishabhverma895334 | 01 Mar, 2024, 07:24: AM
CBSE 12-science - Physics
Asked by rameshsanju123 | 08 Feb, 2024, 08:45: PM
CBSE 12-science - Physics
Asked by sachin.sondur2012 | 07 Feb, 2024, 11:26: AM











