JEE Class main Answered
Write the Lewis dot structure of CO3-2 and explain with steps.
Asked by ojili005 | 29 May, 2021, 08:26: PM
Lewis dot structure-
-in this structure, C is central atom and number of electrons in C is 4.
-Two Oxygen are attached to carbon.
-O has 6 electrons so each oxygen will share 2 electron with carbon to attain stability.
By following these p[onts we can make structures like this-
-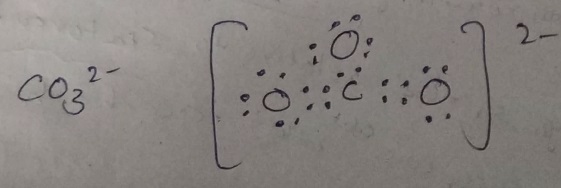

Here -2 charge means one O atom gained 2 electrons.
Answered by Ravi | 31 May, 2021, 07:01: PM
JEE main - Chemistry
Asked by gattimadhavi434 | 25 Dec, 2023, 10:15: AM
JEE main - Chemistry
Asked by visalvinod85 | 23 Jun, 2022, 08:41: AM
JEE main - Chemistry
Asked by deba.biswas561 | 19 Jun, 2022, 09:00: AM
JEE main - Chemistry
Asked by rakeebalikcl | 13 Jun, 2022, 05:47: AM
JEE main - Chemistry
Asked by pachchigarkeyur | 25 Mar, 2022, 06:09: PM
JEE main - Chemistry
Asked by pachchigarkeyur | 22 Mar, 2022, 12:37: PM
JEE main - Chemistry
Asked by pachchigarkeyur | 22 Mar, 2022, 12:35: PM
JEE main - Chemistry
Asked by ojili005 | 31 May, 2021, 07:20: PM



