JEE Class main Answered
Where H+ Attack How To Know
Asked by sahusohan179 | 12 Jan, 2023, 05:24: AM
Dear Student,
H+ will attack the molecule at the position whiere electron density is more.
It attackes in such a way that the resulting structure would be more stable.
More the resonance, more the stability.
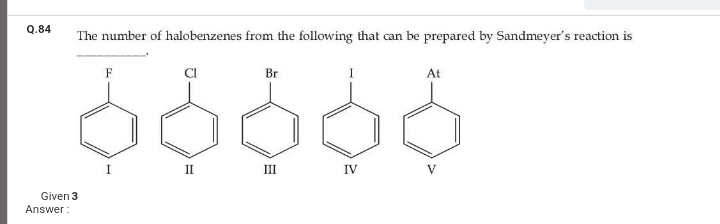
For example,
Answered by | 12 Jan, 2023, 12:37: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 06:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 01:21: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 09:44: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 11:27: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM