JEE Class main Answered
A solution containing
0.85
g
of
Z
n
C
l
2
in
125.0
g
of water freezes at
−
0.23
o
C
. The apparent degree of dissociation of the salt is :
(
k
f
for water=
1.86
K
k
g
m
o
l
−
1
, atomic mass;
Z
n
=
65.3
and
C
l
=
35.5
)
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
Dear Student,
Degree of dissociation of ZnCl2
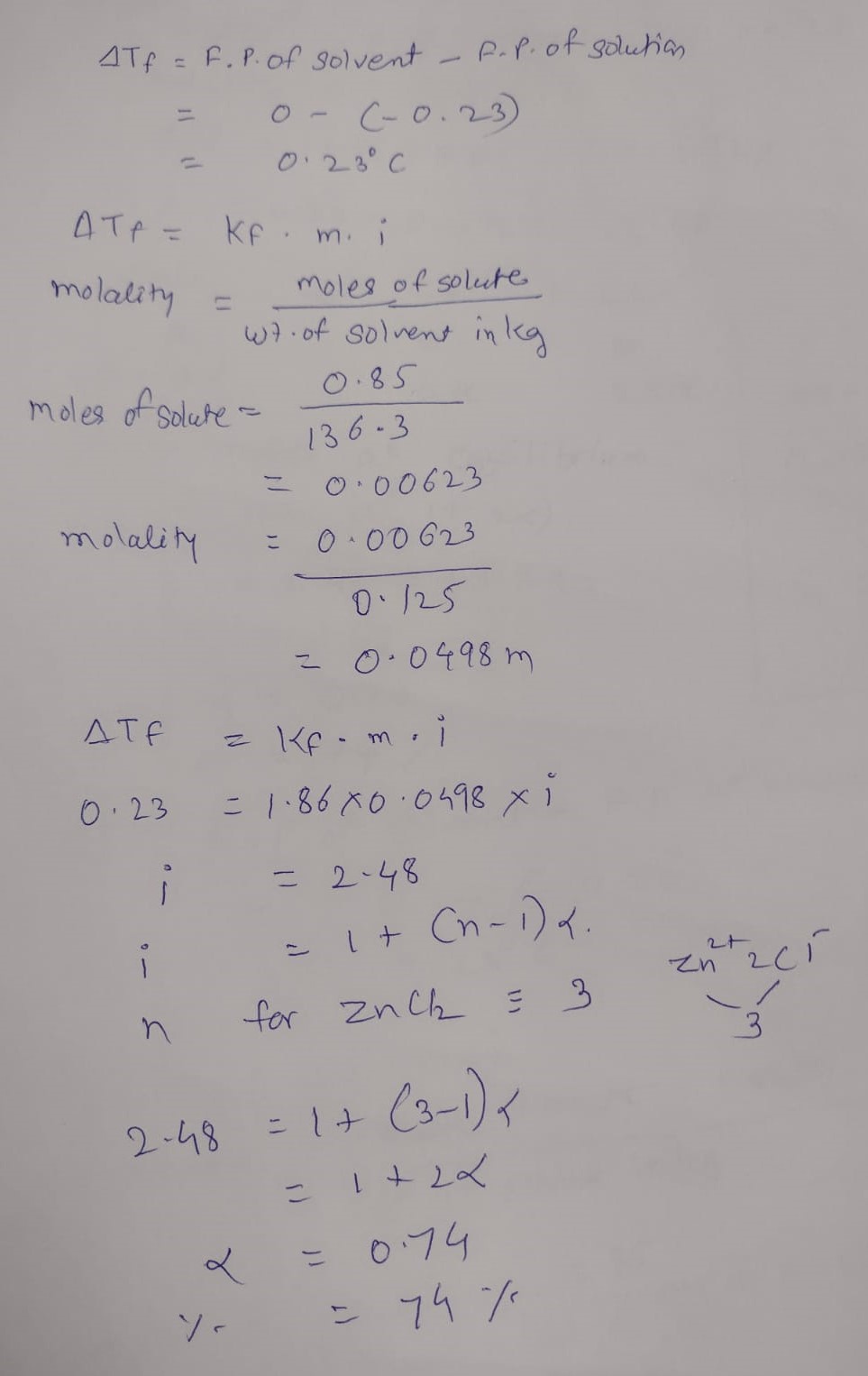
Answered by | 08 Apr, 2024, 12:21: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 08:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 02:23: PM



