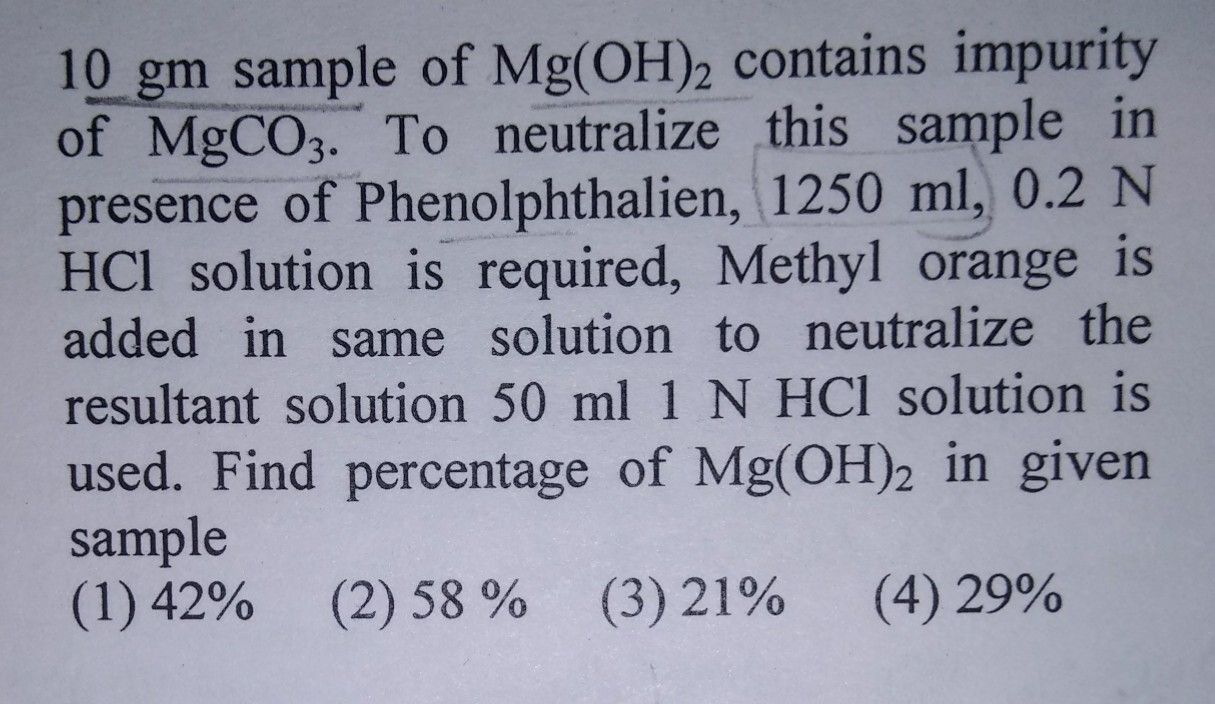
CBSE Class 11-science Answered
total mass of 1 dozen carbon atoms is
Asked by gauravkhandelwal568 | 29 Jun, 2021, 02:31: PM
Number of atoms can be calculated by stoichiometry.
Cehmical equation for this process-

Answered by Ravi | 01 Jul, 2021, 12:53: PM
CBSE 11-science - Chemistry
Asked by brijk456 | 22 Aug, 2019, 11:00: AM
CBSE 11-science - Chemistry
Asked by theraianurag1 | 04 Jun, 2019, 01:00: PM
CBSE 11-science - Chemistry
Asked by Sridhar | 16 May, 2019, 12:05: PM
CBSE 11-science - Chemistry
Asked by easy.shopu | 03 May, 2019, 05:28: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 19 Apr, 2019, 09:46: AM
CBSE 11-science - Chemistry
Asked by Karthika.m.nair01 | 01 Mar, 2019, 11:58: AM