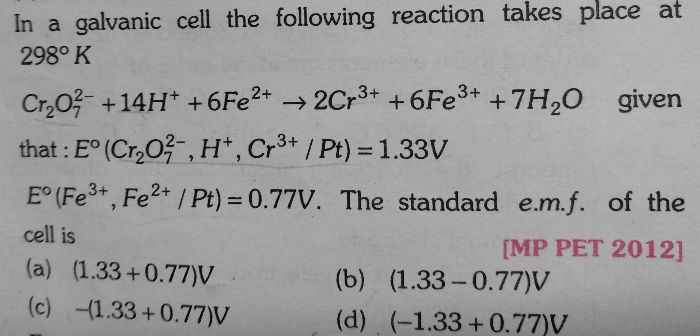NEET Class neet Answered
the passage of current liberates h2 at cathode and cl2 at anode . the solution is
Asked by sr8834055 | 20 Mar, 2024, 02:54: PM
Dear Student,
The solution is sodium chloride in water.
As the discharge potential of water is greater than that of sodium water is reduced at the cathode instead of Na+1.
The reactions at the cathode and anode are as follows:
Cathode: H2O + e- → 1/2 H2 + OH-
Anode: Cl- → 1/2 Cl₂ + e-
Answered by | 21 Mar, 2024, 11:59: AM
NEET neet - Chemistry
Asked by sr8834055 | 20 Mar, 2024, 02:54: PM
NEET neet - Chemistry
Asked by dshreya247 | 03 Feb, 2024, 11:32: AM
NEET neet - Chemistry
Asked by sujitjana971 | 15 Dec, 2022, 08:09: PM
NEET neet - Chemistry
Asked by zoyakhan98264 | 16 Jul, 2022, 01:59: PM
NEET neet - Chemistry
Asked by gurugubellisaivishal2705 | 30 Jun, 2022, 12:32: PM
NEET neet - Chemistry
Asked by ansh.bharso | 28 Jun, 2022, 03:33: PM
NEET neet - Chemistry
Asked by dev28011997 | 09 Oct, 2021, 02:21: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:43: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:39: PM