JEE Class main Answered
Sir plz solve it step by step,plzz
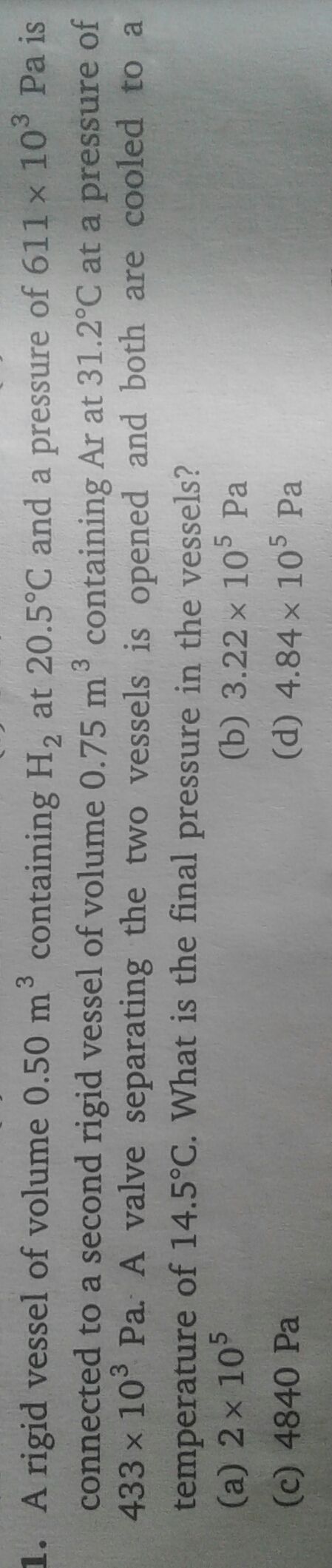
Asked by vishakhachandan026 | 30 Jul, 2019, 06:28: PM
Given:
For hydrogen,
Volume = 0.5 m3
Temperature = 20.5 °C
=273 + 20.5 = 293.5 K
Pressure = 611 × 103 Pa
Calculate no.of moles by using gas law,
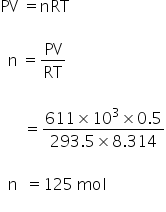
For argon,
Volume = 0.750 m3
Temperature =31.2 °C
= 273 + 31.2=304.2 K
Pressure = 433 × 103 Pa
Calculate no.of moles by using gas law,
PV =nRT

Total no. of moles = 125+128 = 253 mole
Final temperature = 14.5 °C
Final volume = 0.5 +0.75 = 1.25 m3
= 273 + 14.5 =287.5 K K
PV = nRT

The final pressure of the vessle is 4.837 × 105 Pa
Answered by Varsha | 02 Aug, 2019, 12:30: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 06:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 02:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM
JEE main - Chemistry
Asked by vuppulojusaritha | 05 Nov, 2023, 02:22: PM
JEE main - Chemistry
Asked by radheshyambaheti085 | 09 Aug, 2023, 07:10: AM
JEE main - Chemistry
Asked by | 17 Aug, 2022, 08:10: PM
JEE main - Chemistry
Asked by aryankatiyar223 | 10 Aug, 2022, 11:57: PM












