CBSE Class 11-science Answered
Sir please teach me how to draw resonance structures
Asked by | 16 Mar, 2013, 10:29: AM
In certain cases, molecules can be represent by more than one reasonable Lewis structure that differ only in the location of ? electrons.
Electrons in ? bonds have a fixed location and so they are said to be localised.
In contrast, ? electrons that can be drawn in different locations are said to be delocalised.
Collectively these Lewis diagrams are then known as resonance structures or resonance contributors or resonance canonicals.
The "real" structure has characteristics of each of the contributors, and is often represented as the resonance hybrid (think of a hybrid breed which is a mixed breed). In a way, the resonance hybrid is a mixture of the contributors.
Electrons in ? bonds have a fixed location and so they are said to be localised.
In contrast, ? electrons that can be drawn in different locations are said to be delocalised.
Collectively these Lewis diagrams are then known as resonance structures or resonance contributors or resonance canonicals.
The "real" structure has characteristics of each of the contributors, and is often represented as the resonance hybrid (think of a hybrid breed which is a mixed breed). In a way, the resonance hybrid is a mixture of the contributors.
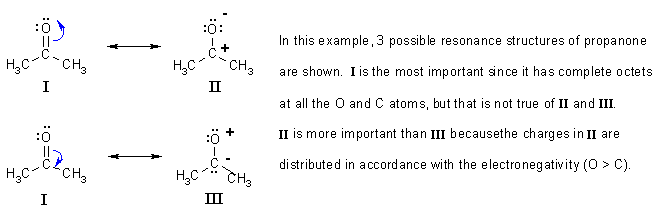
Answered by | 16 Mar, 2013, 06:48: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM






