JEE Class main Answered
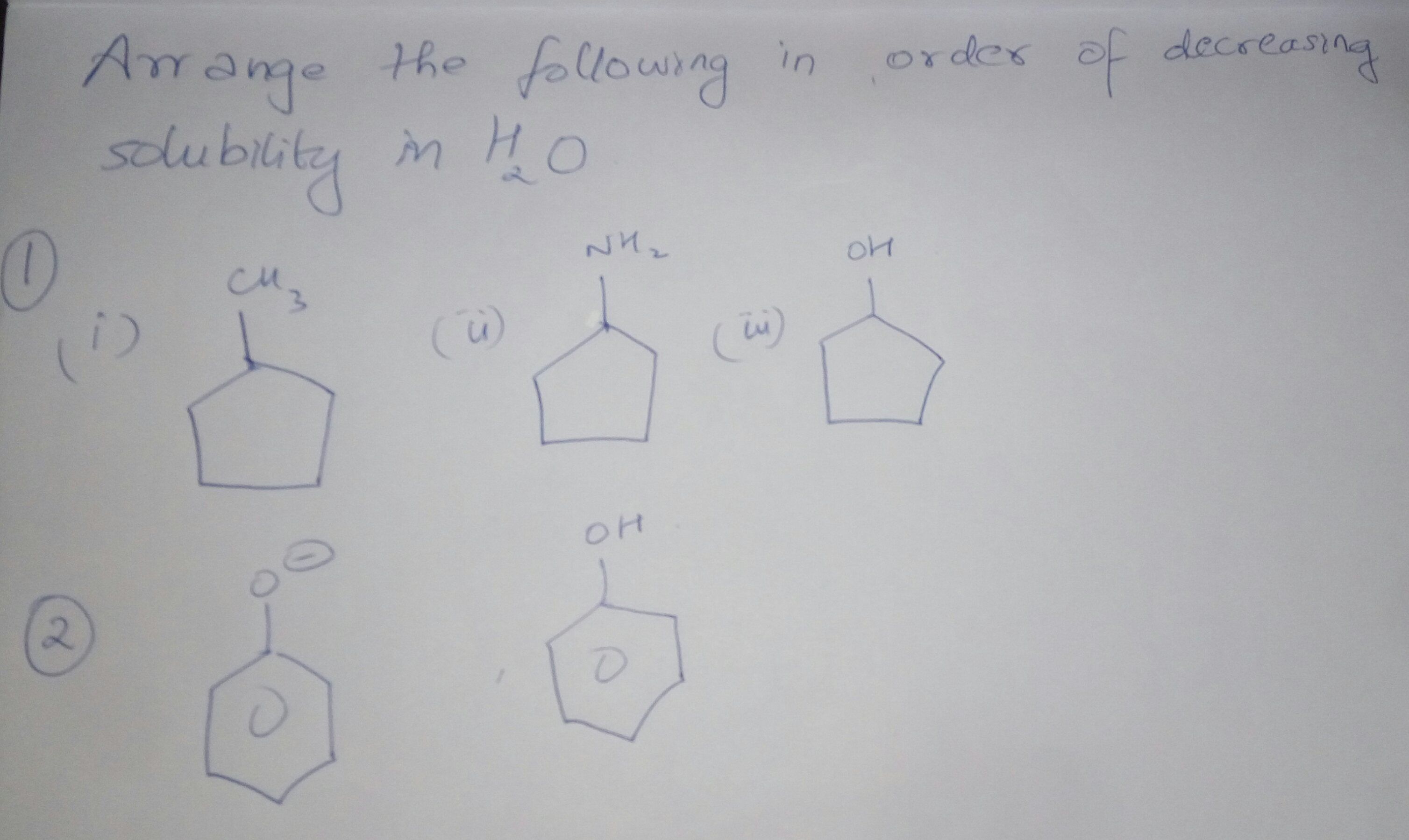
Sir, in question (1) , answer is given as (ii)> (iii)> (i) and in question (2), the answer is given as (i) > (ii) . Please explain how because I feel that answer of question (1) should be (iii) > (ii) > (i) and answer of question (2) should be (ii) > (i) because of hydrogen bonding.
Asked by sumayiah2000 | 12 May, 2019, 08:18: PM
1) N being less electronegative than O atom so lone pair electrons of N will form more effective H Bond and thus solubility will be high, thus the order is ii>iii>i
2) In case of phenoxide ion complete -ve charge is present on atom thus it will strongly attract the proton from water which will elevate the extent of formation of H bonding and will increase the solubility, thus answer is i>ii
Answered by Sumit Chakrapani | 13 May, 2019, 12:26: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 01:21: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 09:44: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 11:27: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by syamalanandini49 | 19 Mar, 2024, 11:58: AM