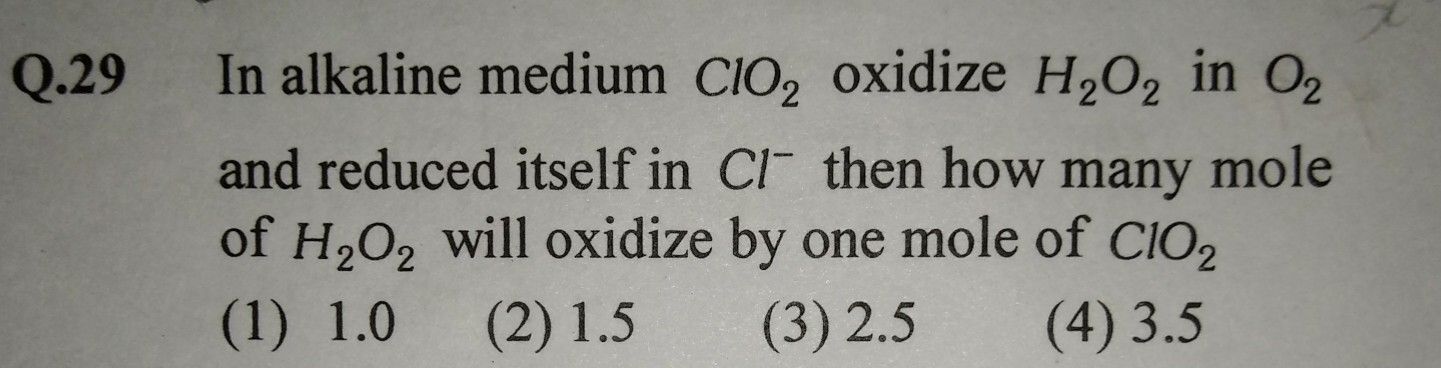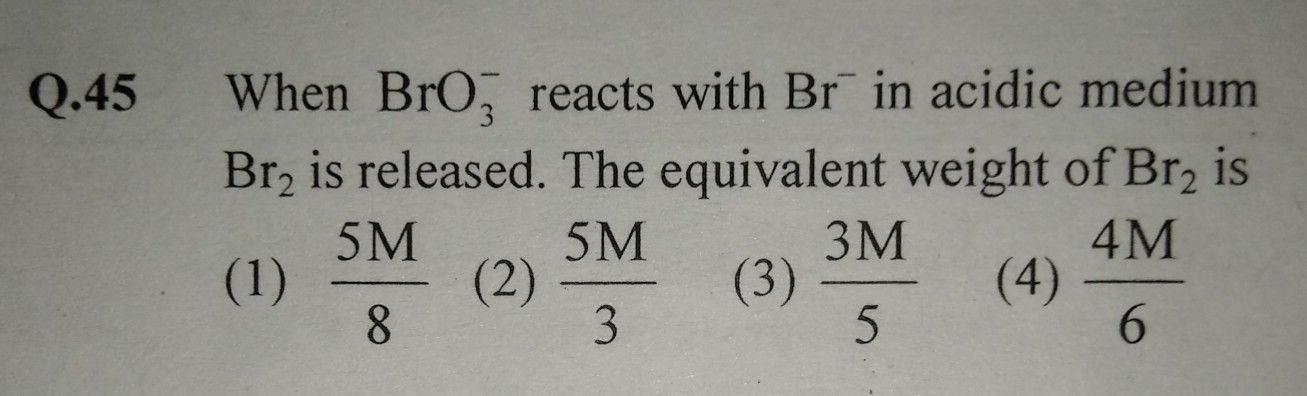NEET Class neet Answered
Same moles of which of the following species will require minimum amount of acidified KMnO4 for complete oxidation?
(1) Ferrous ion
(2) Sulphide ion
(3) Oxalate ion
(4) Sulphite ion
Please solve it. Also is there any shortcut to solve this question fast.
Asked by rohitraman1115 | 22 May, 2021, 04:13: PM
Oxidation number of Mn in KMnO4 is +7 and it is reduced to +5.

Answered by Ravi | 22 May, 2021, 10:23: PM
NEET neet - Chemistry
Asked by shubhisingh20001 | 08 Nov, 2023, 01:17: PM
NEET neet - Chemistry
Asked by ssolaimuthu9 | 08 Jul, 2022, 01:01: PM
NEET neet - Chemistry
Asked by rohitraman1115 | 22 May, 2021, 04:13: PM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:11: PM
NEET neet - Chemistry
Asked by anjanakurup728 | 21 Nov, 2019, 10:06: AM
NEET neet - Chemistry
Asked by sumayiah2000 | 20 Nov, 2019, 08:09: PM
NEET neet - Chemistry
Asked by Balbir | 28 Aug, 2019, 07:52: PM
NEET neet - Chemistry
Asked by ctmonasara914 | 27 Aug, 2019, 11:01: PM
NEET neet - Chemistry
Asked by brijk456 | 13 Aug, 2019, 11:46: PM