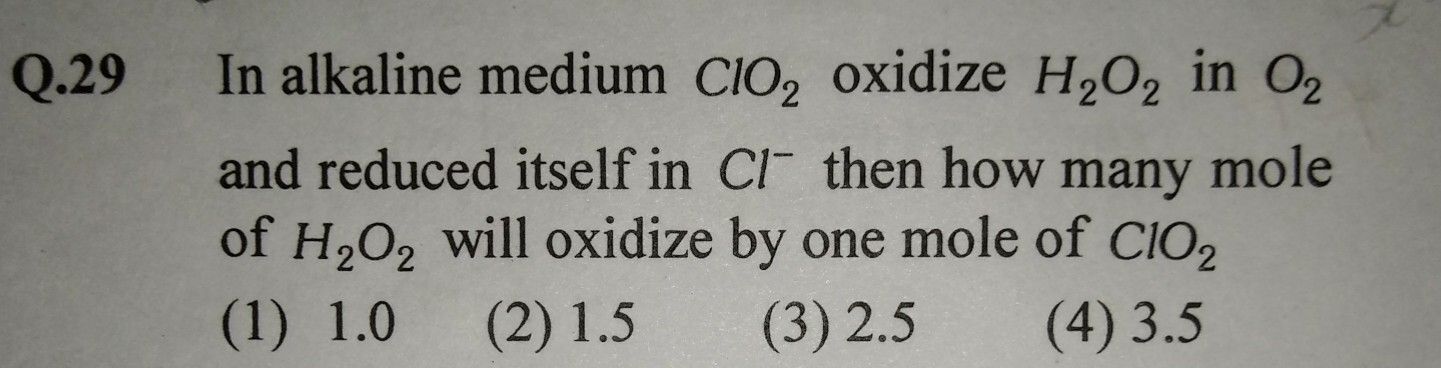NEET Class neet Answered
the equivalent weight of the h3po3, when it disproportionate into the and h3po3 is
Asked by ssolaimuthu9 | 08 Jul, 2022, 01:01: PM
The balanced disproportionation reaction of H3PO2 is,
3H3PO2 → PH3 + 2H3PO3
“n” factor is total change in oxidation state.
Equivalent weight = Molecular weight / n factor
In the change of H3PO2 → H3PO3
The oxidation state of P changes from +1 to +3.
P+1 + 4e− → P+3
So, n = 4
Eq. W = M/4
While in the change of H3PO2 → PH3
The oxidation state of P changes from +1 to -3.
P+1 → P+3 + 2e−
So, n = 2
Eq. W = M/2
Eq. wt. of H3PO2 = (M/4) + (M/2) = 3M/4
Answered by | 08 Jul, 2022, 02:57: PM
NEET neet - Chemistry
Asked by shubhisingh20001 | 08 Nov, 2023, 01:17: PM
NEET neet - Chemistry
Asked by ssolaimuthu9 | 08 Jul, 2022, 01:01: PM
NEET neet - Chemistry
Asked by rohitraman1115 | 22 May, 2021, 04:13: PM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:11: PM
NEET neet - Chemistry
Asked by anjanakurup728 | 21 Nov, 2019, 10:06: AM
NEET neet - Chemistry
Asked by sumayiah2000 | 20 Nov, 2019, 08:09: PM
NEET neet - Chemistry
Asked by Balbir | 28 Aug, 2019, 07:52: PM
NEET neet - Chemistry
Asked by ctmonasara914 | 27 Aug, 2019, 11:01: PM
NEET neet - Chemistry
Asked by brijk456 | 13 Aug, 2019, 11:46: PM