JEE Class main Answered
Q) 3.55 gm of bleaching powder is suspended in water and treated with dilute H2SO4 and excess of potassium iodide liberated iodine just requires 80 ml of iodine solution calculate percentage av of chlorine in bleaching powder.
Asked by Anish | 01 May, 2019, 01:27: PM
Question:
3.55 g sample of bleaching powder suspended in H2 O was treated with dil.H2SO4 and KI solution. Iodine thus liberated requires 80 mL of 0.2 M hypo for titration. Calculate the % of available chlorine in bleaching powder.
Answer:
Bleaching powder + Acid + KI → KI3 + starch (hypo) → end point (Blue →colourless)
Formula:
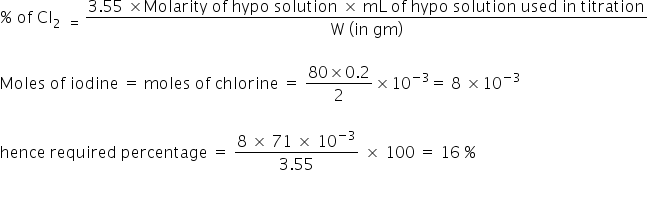
Answered by Ramandeep | 02 May, 2019, 01:01: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 06:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 02:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM
JEE main - Chemistry
Asked by vuppulojusaritha | 05 Nov, 2023, 02:22: PM
JEE main - Chemistry
Asked by radheshyambaheti085 | 09 Aug, 2023, 07:10: AM
JEE main - Chemistry
Asked by | 17 Aug, 2022, 08:10: PM
JEE main - Chemistry
Asked by aryankatiyar223 | 10 Aug, 2022, 11:57: PM




