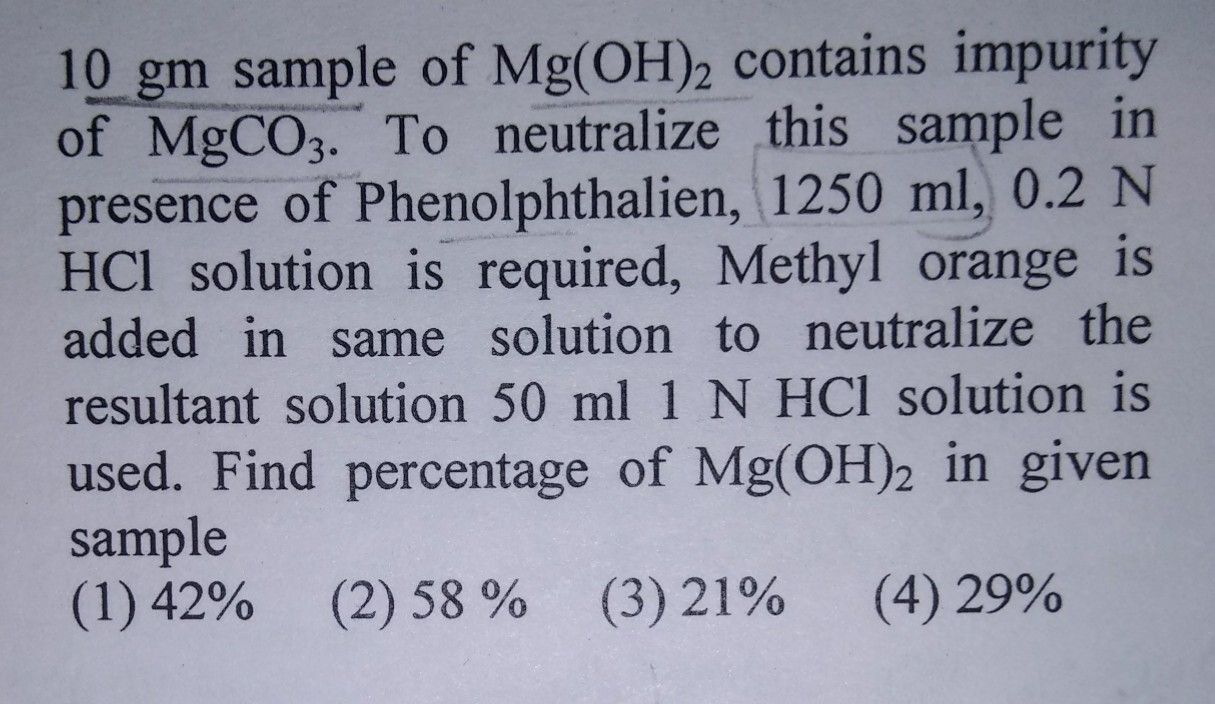
CBSE Class 11-science Answered
please answer this

Asked by Prashant DIGHE | 29 Feb, 2020, 09:49: PM
According to law of chemical equivalence,
gEq (M) = gEq (Ag)
{ 0.6538 × nM}/M = {2.16 × nAg}/MAg
{0.6538 × 2 }/M = {2.16 × 1}/108
So, 2.16M = 141.22
M = 65.38
Answered by Ravi | 01 Mar, 2020, 08:44: PM
CBSE 11-science - Chemistry
Asked by brijk456 | 22 Aug, 2019, 11:00: AM
CBSE 11-science - Chemistry
Asked by theraianurag1 | 04 Jun, 2019, 01:00: PM
CBSE 11-science - Chemistry
Asked by Sridhar | 16 May, 2019, 12:05: PM
CBSE 11-science - Chemistry
Asked by easy.shopu | 03 May, 2019, 05:28: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 19 Apr, 2019, 09:46: AM