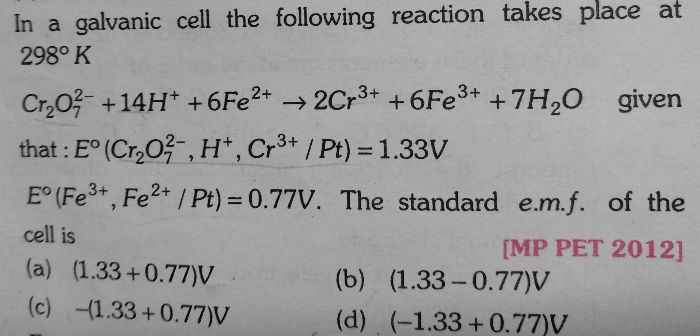NEET Class neet Answered
Please annswer the following question.
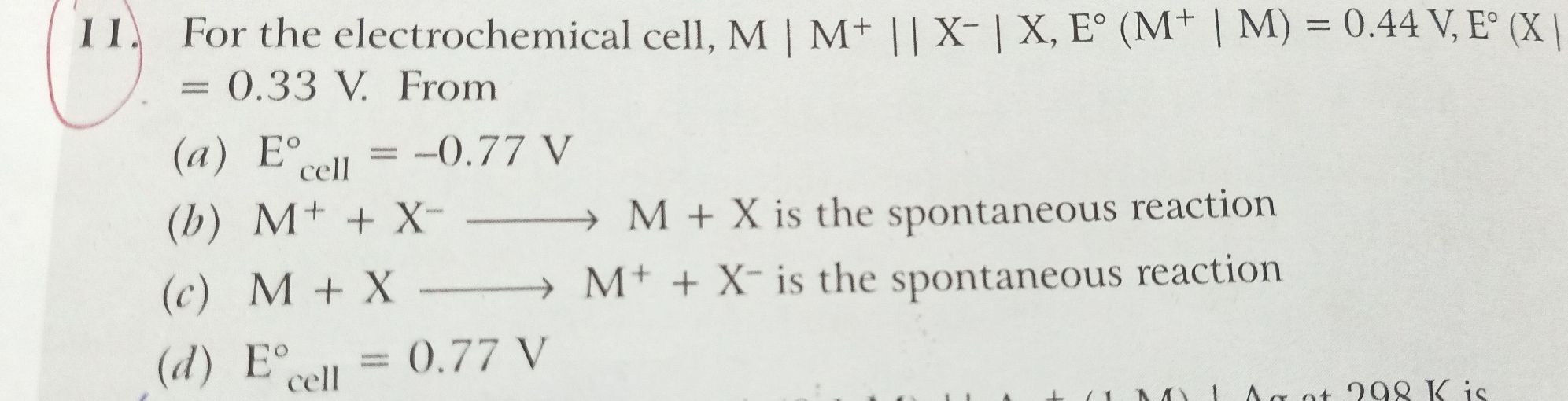
Asked by Balbir | 28 Aug, 2019, 08:33: PM
Given:
M+ + X− → M + X is spontaneous reaction.
The cell reaction is,
X + e− → X−
M → M+ + e−
Overall reaction is,
X + M → M+ + X−
By using Nernst Equation,
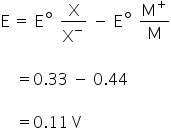
We know that,
ΔG = −nFE
When value of ΔG is negative, the reaction is spontaneous.
Here, E = 0.11 V
ΔG = −nF × 0.11
Which will be negative value
So the it is spontaneous reaction.
Answered by Varsha | 29 Aug, 2019, 06:01: PM
NEET neet - Chemistry
Asked by sr8834055 | 20 Mar, 2024, 02:54: PM
NEET neet - Chemistry
Asked by dshreya247 | 03 Feb, 2024, 11:32: AM
NEET neet - Chemistry
Asked by sujitjana971 | 15 Dec, 2022, 08:09: PM
NEET neet - Chemistry
Asked by zoyakhan98264 | 16 Jul, 2022, 01:59: PM
NEET neet - Chemistry
Asked by gurugubellisaivishal2705 | 30 Jun, 2022, 12:32: PM
NEET neet - Chemistry
Asked by ansh.bharso | 28 Jun, 2022, 03:33: PM
NEET neet - Chemistry
Asked by dev28011997 | 09 Oct, 2021, 02:21: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:43: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:39: PM