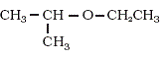CBSE Class 12-science Answered
On what basis does the R-O Cleavage takes place when R-O-R reacts with HI ?
eg-: CH3-CH2-CH2-O-CH3 + HI ---> CH3-CH2-CH2-OH + CH3I ,
Why is the product not CH3-CHI-CH3 + CH4 ?
eg-: CH3-CH2-CH2-O-CH3 + HI ---> CH3-CH2-CH2-OH + CH3I ,
Why is the product not CH3-CHI-CH3 + CH4 ?
Asked by Rohit Virinchi | 11 Mar, 2015, 02:41: PM
The clevage of ethers by halogen acids occurs by the following mechanism:
step 1: Ethers being Lewis bases, undergo protonation to form oxonium ions. In this compound the oxonium ion formed is given below.
Step 2: Iodide ion is a good nucleophile. Due to steric hindrance, it attacks the smaller alkyl group of the oxonium ion formed in step 1 and displaces the alcohol molecule by SN2 mechanism as shown below:
In the given molecule, methyl group is smaller alkyl group, hence methyl iodide is formed.
Answered by Prachi Sawant | 12 Mar, 2015, 12:22: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 04:55: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 13 Jan, 2020, 09:34: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 12 May, 2019, 05:47: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:22: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:22: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:43: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:49: AM