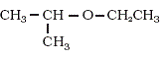CBSE Class 12-science Answered
Answer 5th with reaction

Asked by lovemaan5500 | 13 Jan, 2020, 09:34: PM
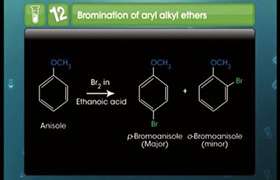
In case one of the alkyl group is a tertiary group in an asymmetrical ether, the halide formed is a tertiary halide.
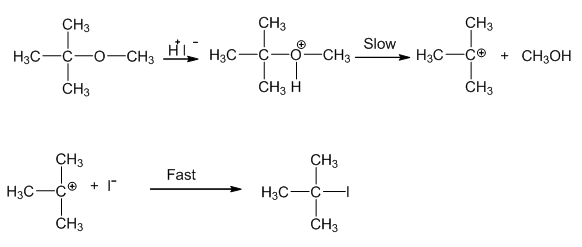

This is because in step 2 when after the breaking of tert-carbon and -OCH3 bond, there is the formation of a more stable carbocation[(CH3)3C+], and the reaction proceeds via SN1 reaction mechanism.
Hence option 4 is the correct answer.
Answered by Ramandeep | 14 Jan, 2020, 01:07: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 04:55: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 13 Jan, 2020, 09:34: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 12 May, 2019, 05:47: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:22: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:22: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:43: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:49: AM