JEE Class main Answered
How many gram of sulfuric acid is required to produce 20g of hydrogen?
Asked by ayushmishradbb | 21 Nov, 2020, 01:38: PM
20 gram of hydrogenb means 20/2=10 moles of H2 .
We know that 2 moles of hydrogen is produced from one mole.
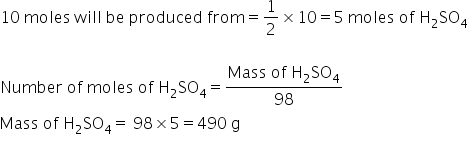
Answered by Ravi | 21 Nov, 2020, 04:51: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 06:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 02:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM
JEE main - Chemistry
Asked by vuppulojusaritha | 05 Nov, 2023, 02:22: PM
JEE main - Chemistry
Asked by radheshyambaheti085 | 09 Aug, 2023, 07:10: AM
JEE main - Chemistry
Asked by | 17 Aug, 2022, 08:10: PM
JEE main - Chemistry
Asked by aryankatiyar223 | 10 Aug, 2022, 11:57: PM




