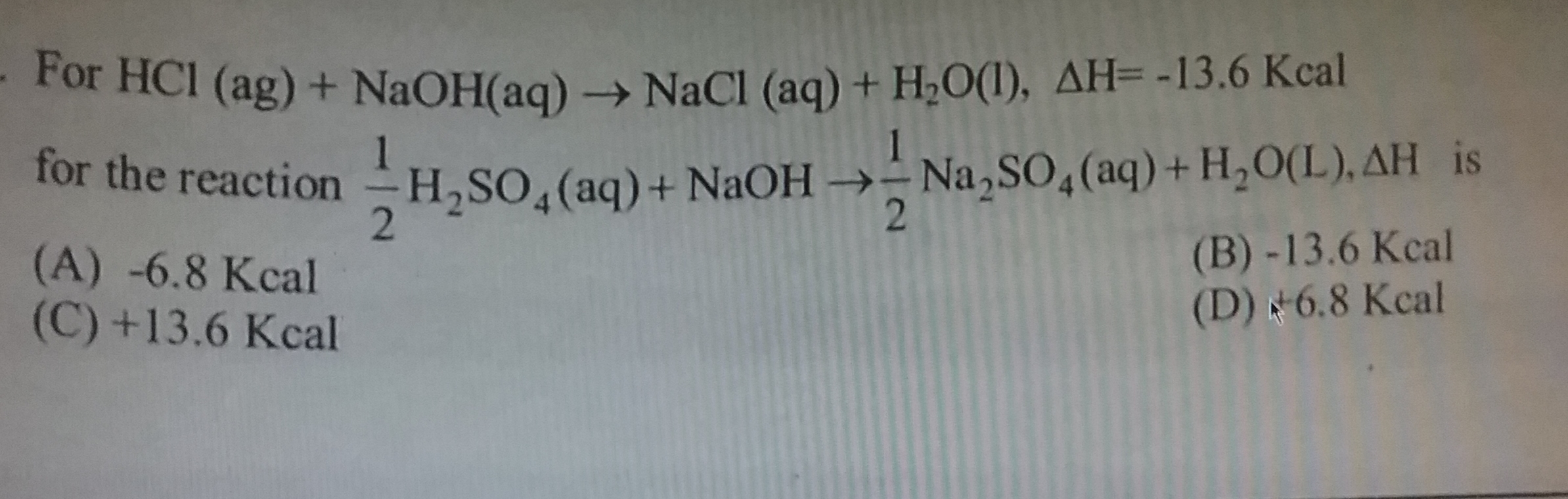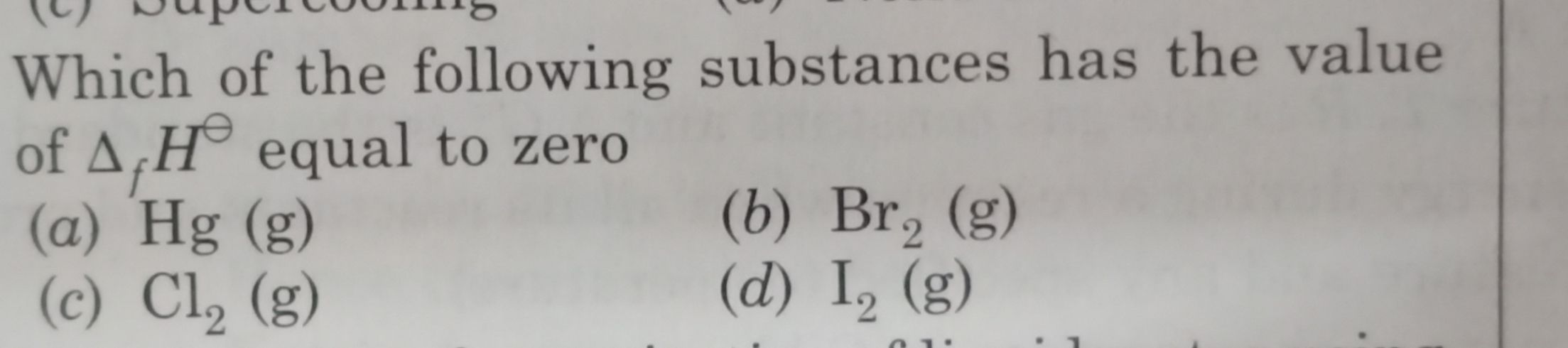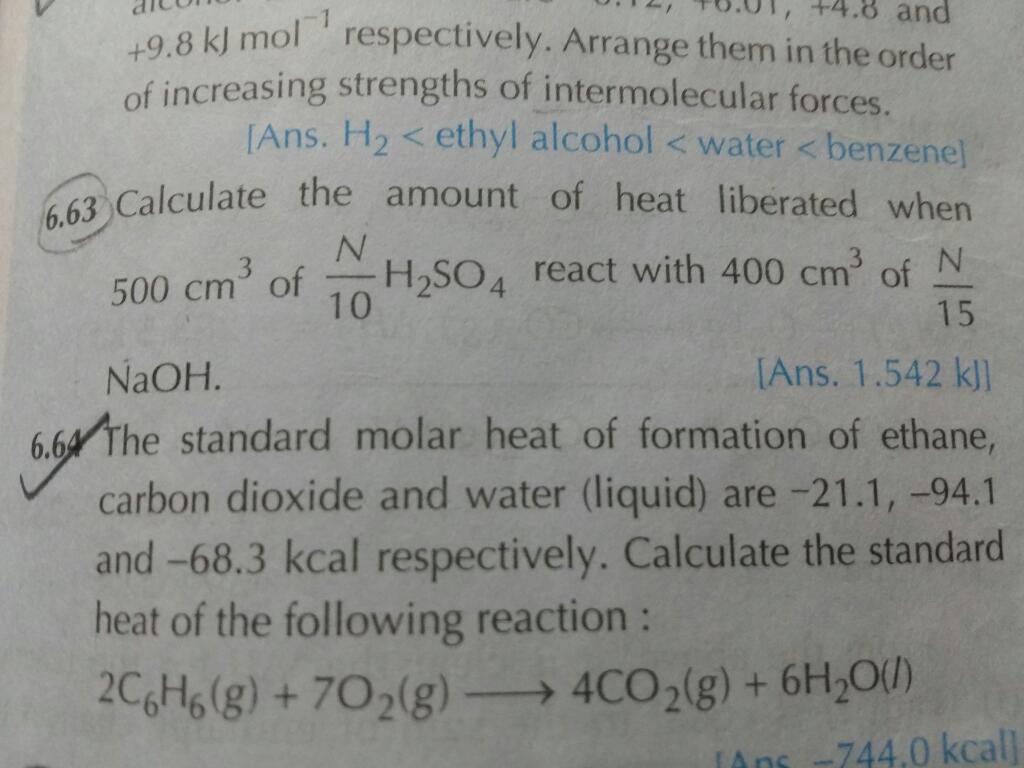CBSE Class 11-science Answered
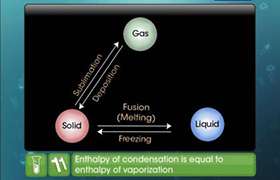
How does the intermolecular interaction affect the magnitude of enthalpy change during phase transformation?
Asked by Topperlearning User | 14 Aug, 2014, 12:45: PM
The strength of intermolecular interaction determines the magnitude of enthalpy change. For example in water there is strong intermolecular interaction in the form of hydrogen bond and in acetone the intermolecular interaction is weak dipole-dipole interaction. Hence enthalpy of vaporization is greater in case of water.
Answered by | 14 Aug, 2014, 02:45: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM