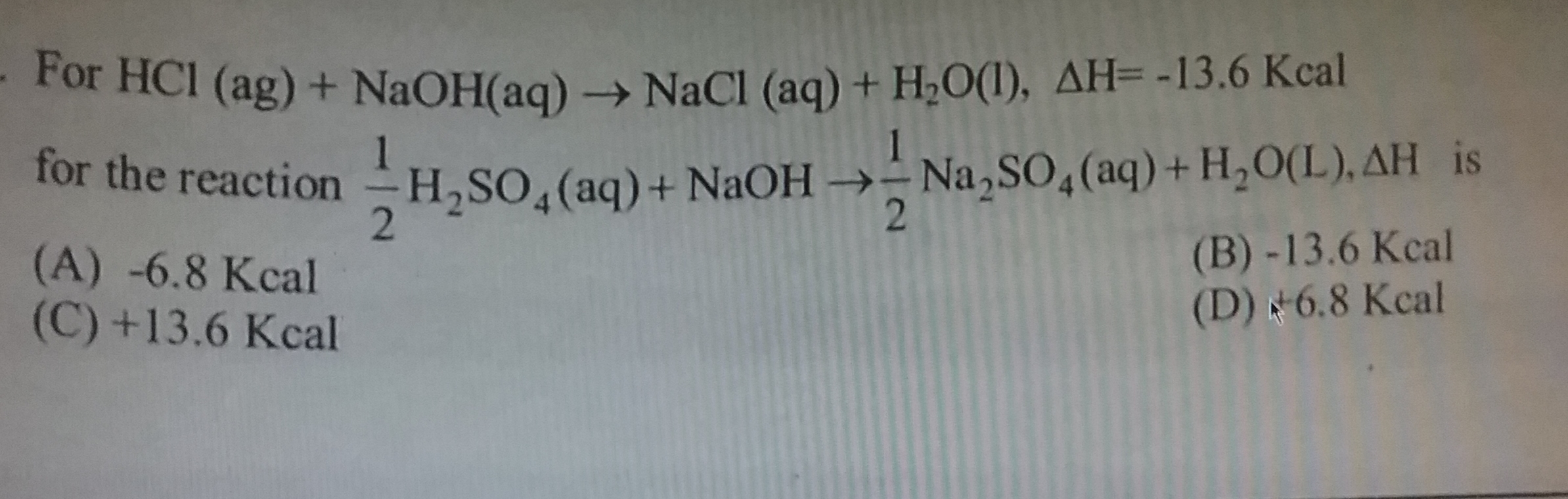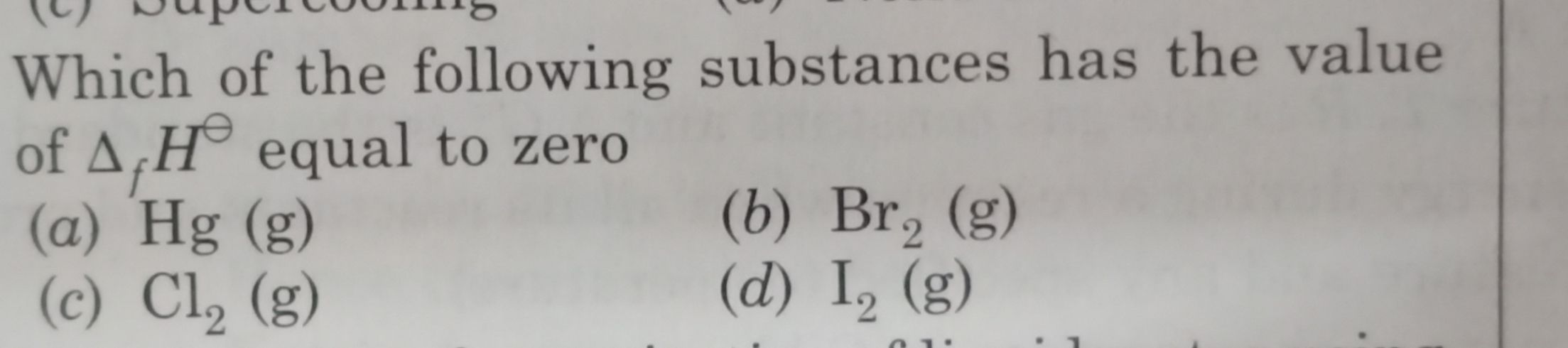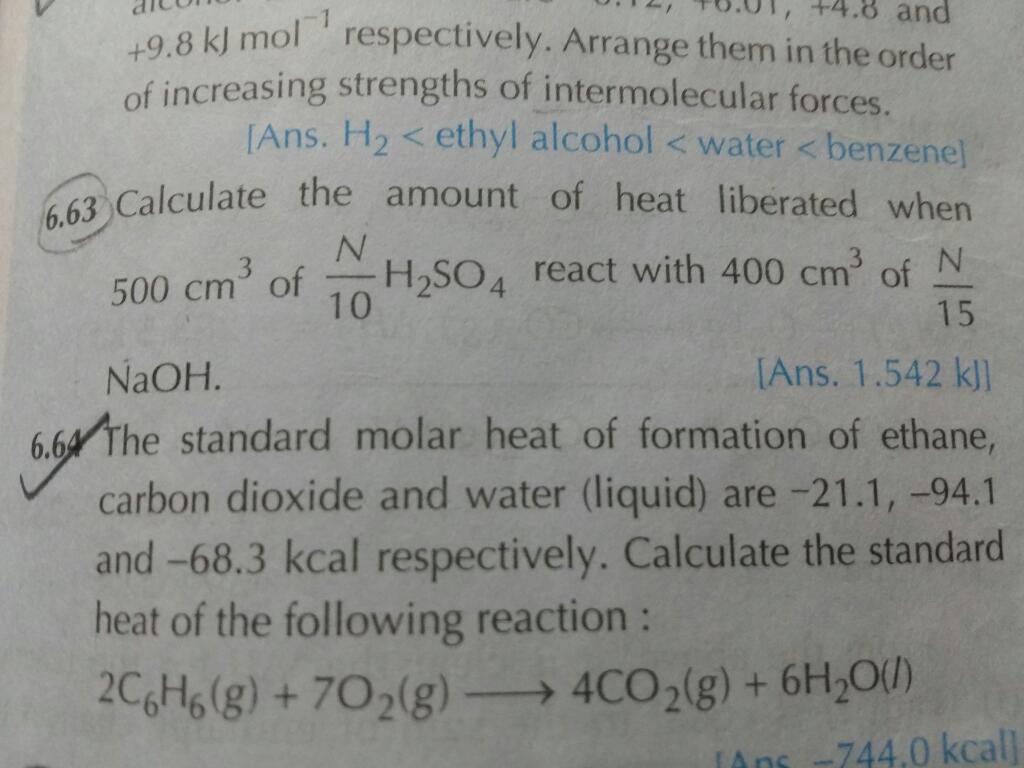CBSE Class 11-science Answered
a steel fiber is in a oxygen filled container which is closed with a frictionless piston. the iron in the steel fiber reacts with oxygen to form Fe2O3 , the heat generated by the reaction is removed during the process to maintain the temperature constant which is 25 degrees centigrade. for the reaction of 2 moles of iron 831.08KJ of heat is removed. calculate the heat , work and internal energy of change of the system
Asked by pranavisrihari | 08 Sep, 2020, 17:24: PM
In this condition tempereature is constant so,
Change in internal energy =0
and we know, accoridng to first law of thermodynamics,
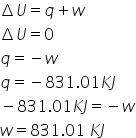
Answered by Ravi | 09 Sep, 2020, 10:18: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 19:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 15:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 17:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 16:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 17:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:24: AM