JEE Class main Answered
Find molality of pure water.
Asked by surajkumar30769 | 12 Aug, 2019, 12:35: PM
We know,
Molar mass of water = 18 gm/mol
Density of water = 1 kg/L
Mass of water 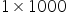
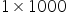
= 1kg
Number of moles of water is,
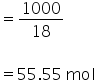
Molality is given by,

Molality of pure water is 55.55 m.
Answered by Varsha | 12 Aug, 2019, 01:04: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 06:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 02:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM
JEE main - Chemistry
Asked by vuppulojusaritha | 05 Nov, 2023, 02:22: PM
JEE main - Chemistry
Asked by radheshyambaheti085 | 09 Aug, 2023, 07:10: AM
JEE main - Chemistry
Asked by | 17 Aug, 2022, 08:10: PM
JEE main - Chemistry
Asked by aryankatiyar223 | 10 Aug, 2022, 11:57: PM












