NEET Class neet Answered
CH3COOH(aq) → CH3COO- (aq) + H+ (aq), ΔrH° = 0.005 kcal g-1
Enthalpy change when 1 mole of Ca(OH)2, a strong base, is completely neutralised by CH3COOH (aq) in dilute solution is
a)
-27.4 kcal mol-1
b)
-13.4 kcal mol-1
c)
-26.8 kcal mol-1
d)
-27.1 kcal mol-1
Correct answer is option 'C'. Can you explain this answer?
Asked by ntg432000 | 06 Mar, 2019, 11:26: AM
the change in enthalpy is calculated as follows:
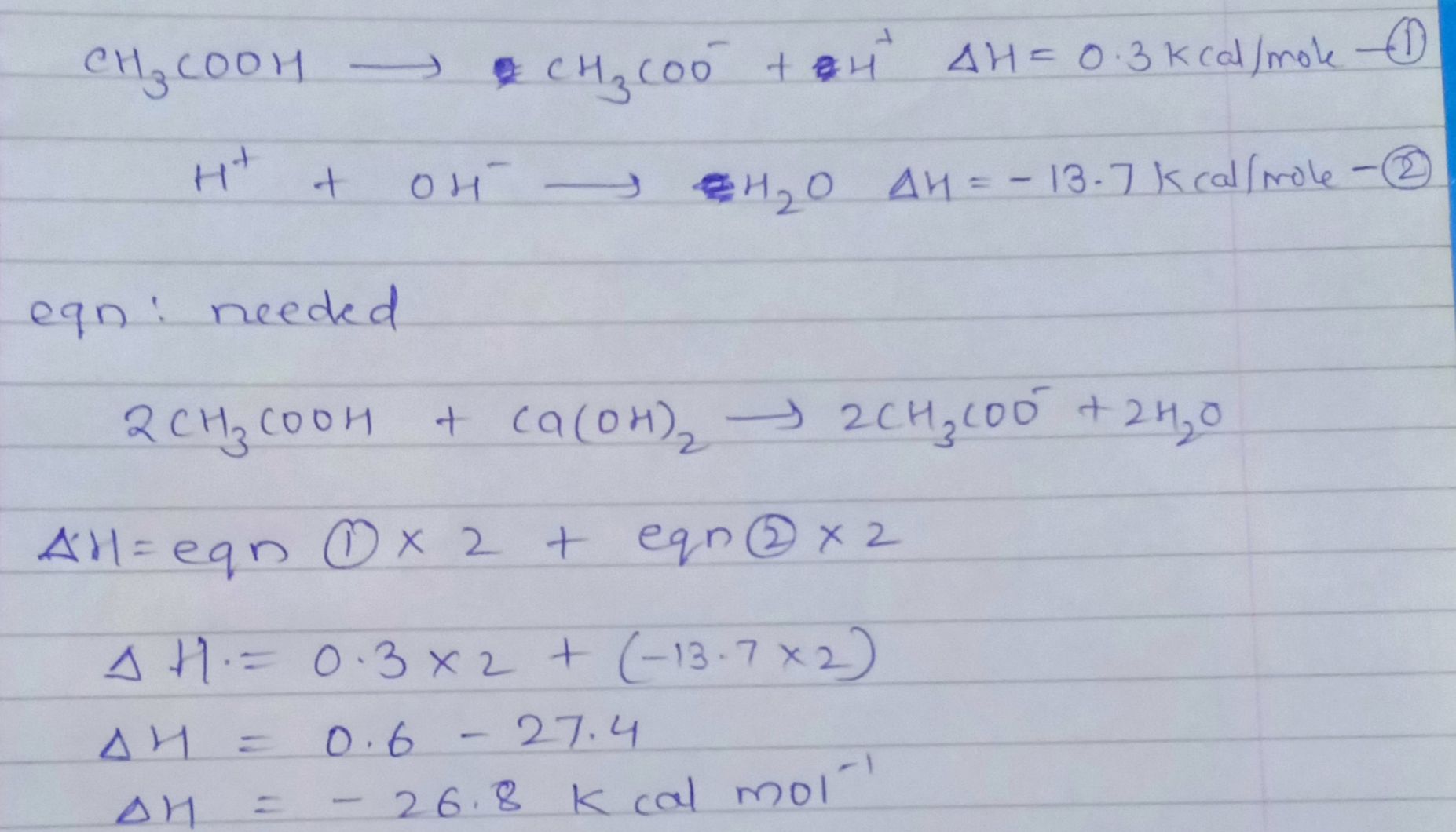
Answered by Ramandeep | 06 Mar, 2019, 06:35: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 Aug, 2021, 04:10: AM
NEET neet - Chemistry
Asked by patra04011965 | 12 Nov, 2019, 09:21: AM
NEET neet - Chemistry
Asked by ntg432000 | 06 Mar, 2019, 11:26: AM