NEET Class neet Answered
⭕ Explain this statement in detail, Thanks 😊
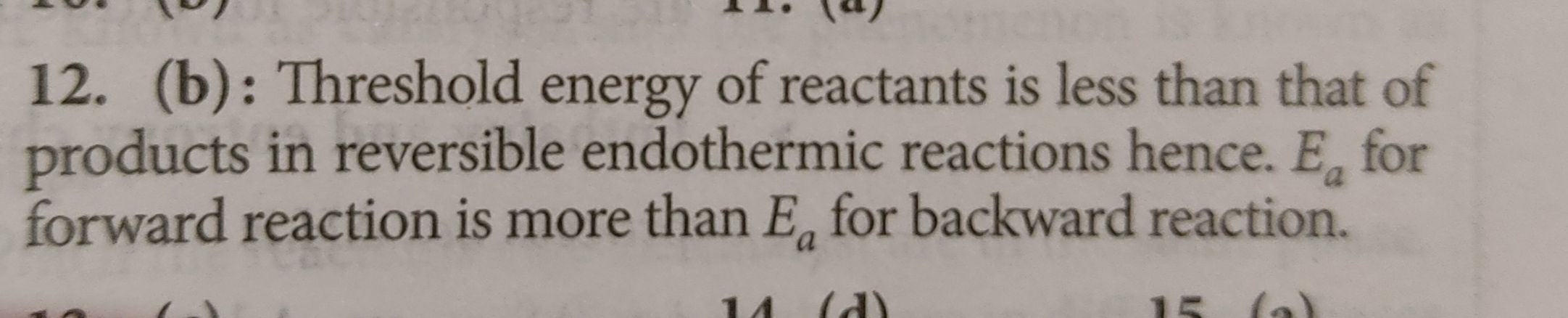
Asked by jhajuhi19 | 10 Aug, 2021, 04:10: AM
Dear Student,
Enthalpy of reaction is calculated by-

So, YOu can see that activation energy of forward reaction is more than activation energy of backward reaction in endothermic reaction.
Answered by Ravi | 11 Aug, 2021, 04:17: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 Aug, 2021, 04:10: AM
NEET neet - Chemistry
Asked by patra04011965 | 12 Nov, 2019, 09:21: AM
NEET neet - Chemistry
Asked by ntg432000 | 06 Mar, 2019, 11:26: AM



