CBSE Class 12-science Questions and Answers
CBSE 12-science - Maths
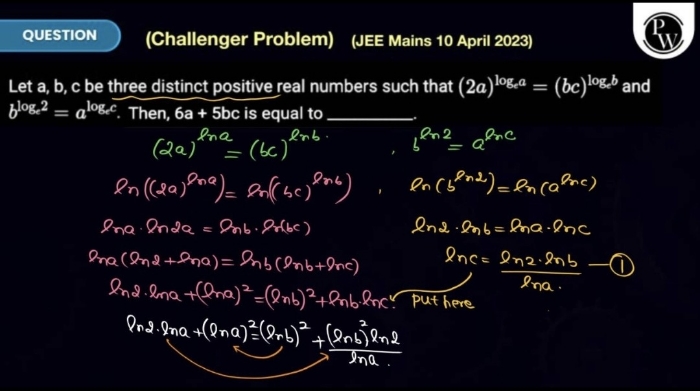
Asked by Karanram4676 | 11 May, 2024, 10:32: PM
CBSE 12-science - Physics
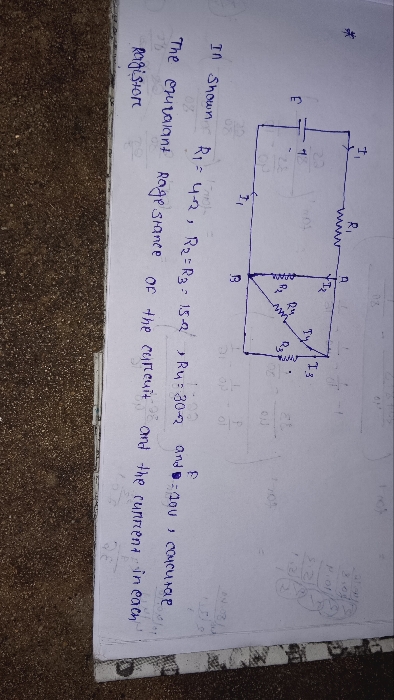
Asked by ankush76728 | 06 May, 2024, 04:52: PM
CBSE 12-science - Physics
Asked by ankush76728 | 05 May, 2024, 09:55: PM
CBSE 12-science - Chemistry
Asked by chetanrakshit06 | 05 May, 2024, 02:51: PM
CBSE 12-science - Maths
Asked by rp978841 | 04 May, 2024, 07:37: PM
CBSE 12-science - Physics
Asked by heymindurownbusiness | 04 May, 2024, 11:15: AM
CBSE 12-science - Physics
Asked by talulu | 01 May, 2024, 05:14: PM
CBSE 12-science - Physics
Asked by kanishkg511 | 30 Apr, 2024, 07:25: PM