ICSE Class 9 Answered
Calculate the volume of dry air at S.T.P. that occupies 28cm3 at 14 degree Celsius and 750 mm Hg pressure when saturated with water vapour.The vapour pressure of water at 14 degree Celsius is 12 mm Hg.
Asked by pradeepkumarsamal | 30 Aug, 2018, 09:43: PM
Given:
P2= 760mm Hg
P1 = 750-12= 738 mm Hg
T1= 14°C = 14 + 273 = 287 K
T2= 0°C = 273K
V1=28 cm3
V2= ?
Formula used: 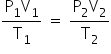
Solution:
Lets put these values in the above formula,
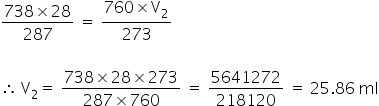
Answered by Ramandeep | 31 Aug, 2018, 10:40: AM
Concept Videos
ICSE 9 - Chemistry
Asked by zainaali39692 | 04 Dec, 2020, 08:53: AM
ICSE 9 - Chemistry
Asked by gup.navya2006 | 01 Dec, 2020, 09:28: AM
ICSE 9 - Chemistry
Asked by Vishusingh2020.2021 | 25 Sep, 2020, 10:09: PM
ICSE 9 - Chemistry
Asked by sudesghnapattanayak2017 | 19 May, 2020, 08:13: PM
ICSE 9 - Chemistry
Asked by abeshchakraborty6 | 23 Feb, 2020, 08:54: AM
ICSE 9 - Chemistry
Asked by dnlwalkers | 08 Jan, 2020, 09:57: AM
ICSE 9 - Chemistry
Asked by raichuratanvi | 14 Dec, 2019, 12:02: PM
ICSE 9 - Chemistry
Asked by merajanjum87 | 21 Nov, 2019, 09:53: PM
ICSE 9 - Chemistry
Asked by parvathimanjunath24 | 31 Oct, 2019, 09:34: PM
ICSE 9 - Chemistry
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM



