ICSE Class 9 Answered
I didn’t understood v vs 1/p graph
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM
Graphical verification of Boyle’s law:
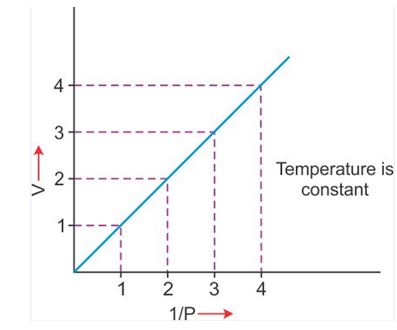
V vs 1/P : Variation in volume (V) plotted against (1/P ) at a constant temperature, a straight line passing through the origin is obtained.
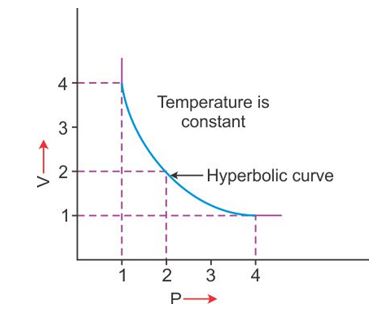
V vs P: Variation in volume (V) plotted against pressure (P) at a constant temperature, a hyperbolic curve in the first quadrant is obtained.
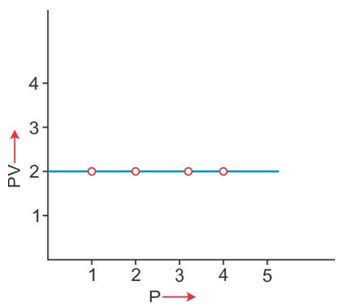
PV vs P: Variation in (PV) plotted against pressure (P) at a constant temperature, a straight line parallel to the X-axis is obtained.
Please refer to the following link for more information:
Answered by Varsha | 23 Sep, 2019, 10:25: AM
Concept Videos
ICSE 9 - Chemistry
Asked by zainaali39692 | 04 Dec, 2020, 08:53: AM
ICSE 9 - Chemistry
Asked by gup.navya2006 | 01 Dec, 2020, 09:28: AM
ICSE 9 - Chemistry
Asked by Vishusingh2020.2021 | 25 Sep, 2020, 10:09: PM
ICSE 9 - Chemistry
Asked by sudesghnapattanayak2017 | 19 May, 2020, 08:13: PM
ICSE 9 - Chemistry
Asked by abeshchakraborty6 | 23 Feb, 2020, 08:54: AM
ICSE 9 - Chemistry
Asked by dnlwalkers | 08 Jan, 2020, 09:57: AM
ICSE 9 - Chemistry
Asked by raichuratanvi | 14 Dec, 2019, 12:02: PM
ICSE 9 - Chemistry
Asked by merajanjum87 | 21 Nov, 2019, 09:53: PM
ICSE 9 - Chemistry
Asked by parvathimanjunath24 | 31 Oct, 2019, 09:34: PM
ICSE 9 - Chemistry
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM