CBSE Class 11-science Answered
Calculate the internal energy of vaporization for 18 g of water at 100°C. Given, 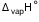 for water at 373 K = 40.66 kJ mol-1.
for water at 373 K = 40.66 kJ mol-1.
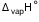 for water at 373 K = 40.66 kJ mol-1.
for water at 373 K = 40.66 kJ mol-1.
Asked by Topperlearning User | 14 Aug, 2014, 01:12: PM
The reaction can be represented as follows
18g H2O (l) 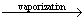 18 g H2O(g)
18 g H2O(g)

Assuming steam behaves as an ideal gas
 = 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1)
= 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1) = 40.66 kJ mol -1 – 3.10 kJ mol -1
= 40.66 kJ mol -1 – 3.10 kJ mol -1 = 37.56 kJ mol -1
Answered by | 14 Aug, 2014, 03:12: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM