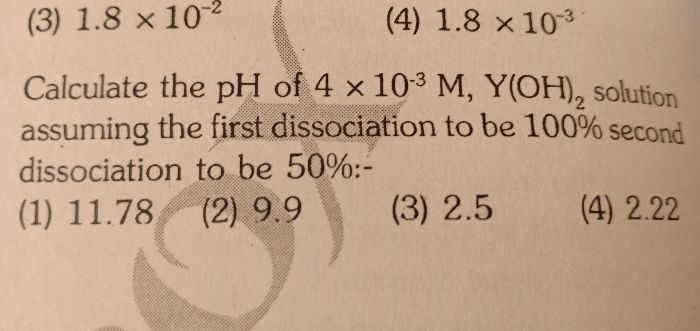CBSE Class 11-science Answered
Calculate the concentration of hydroxyl ion in 0.2 M solution of NH3OH having Kb = 1.8 x 10-4.
Asked by Topperlearning User | 28 Apr, 2015, 10:43: AM
Use Oswald’s Dilution Law formula;
α = √ kb/C
Substitute the respective values; we get
α =√ kb/C = √1.8 x 10-4 /0.2 = .03 = 3%
[OH-] = 0.2 x .03 = 6.0 x 10 -3 M
Answered by | 28 Apr, 2015, 12:43: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:50: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:44: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 14 Apr, 2020, 02:42: PM
CBSE 11-science - Chemistry
Asked by SanskarAgarwal86 | 29 Feb, 2020, 04:36: AM