CBSE Class 9 Answered
(a) Draw a sketch of Bohr's model of an atom with three shells.(b) If K, L and M shell of an atom are full, then what would be the total number of electrons in the atom? (c) What is the maximum number of electrons that can be accommodated in a shell?
Asked by Topperlearning User | 04 Apr, 2016, 10:00: AM
(a)
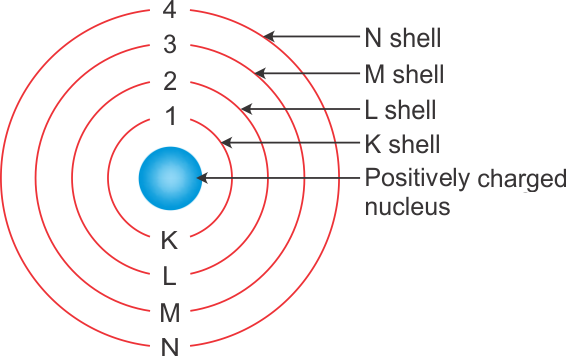
(b) Number of electrons in fully filled K shell = 2
Number of electrons in fully filled L shell = 8
Number of electrons in fully filled M shell = 8
So, total number of electrons = 2 + 8 + 8 = 18
(c) The maximum number of electrons that can be accommodated in a shell = 2n2
where n = shell number.
Answered by | 04 Apr, 2016, 12:00: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by archanakad | 25 Aug, 2020, 08:59: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 15 Apr, 2020, 01:51: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 12 Apr, 2020, 11:47: AM
CBSE 9 - Chemistry
Asked by snaik0856 | 29 Dec, 2019, 09:47: PM
CBSE 9 - Chemistry
Asked by Regiesmalom99 | 18 Oct, 2019, 01:36: PM
CBSE 9 - Chemistry
Asked by Vishalgarg1234 | 25 Nov, 2018, 03:21: PM
CBSE 9 - Chemistry
Asked by hk.sachdev | 12 Oct, 2018, 10:38: PM
CBSE 9 - Chemistry
Asked by sshivali3333 | 19 Sep, 2018, 11:06: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:49: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:50: PM







 . Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you.
. Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you. 