CBSE Class 12-science Answered
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature.What series of wavelengths will be emitted?
Asked by sumitchauhansc20 | 04 Mar, 2015, 06:34: PM
Hi Sumit,
In ground state, energy of gaseous hydrogen at room tempertaure= - 13.6 eV. When bombarded with 12.5 eV electron beam, the energy becomes, -13.6 +12.5 = -1.1 eV
i.e. electron would jump from n=1 to n=3 where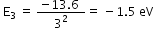
On de-excitation the electron may jump from either n=3 to n=2 givind rise to Balmer series
It may also jump from n = 3 to n =1 , giving rise to Lyman series.
Answered by | 04 Mar, 2015, 06:44: PM
Concept Videos
CBSE 12-science - Physics
Asked by panneer1766 | 24 Apr, 2024, 01:52: PM
CBSE 12-science - Physics
Asked by artabandhusahu85 | 24 Apr, 2024, 12:07: PM
CBSE 12-science - Physics
Asked by niharvijayvargiya5 | 23 Apr, 2024, 06:40: PM
CBSE 12-science - Physics
Asked by kulhariabhijeet | 21 Apr, 2024, 02:39: PM
CBSE 12-science - Physics
Asked by mohapatraswetalina88 | 21 Apr, 2024, 12:18: PM
CBSE 12-science - Physics
Asked by aishaisha091098 | 19 Apr, 2024, 04:54: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 13 Apr, 2024, 06:56: AM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM