CBSE Class 12-science Answered
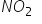 FORMS A DIMER BUT
FORMS A DIMER BUT 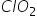 DOES NOT WHY ??
DOES NOT WHY ??
Asked by catherinejohnson2k | 05 Sep, 2016, 10:38: PM
NO2 has an unpaired electron. Hence it behaves as typical odd electron molecule. Because of the presesnce of an unpaired electron, it has a tendency to dimerise to N2O4, with even number of electrons.
ClO2 is also an odd electron molecule, but it is stabilised by resonanace. Hence, it does not undergo dimerisation.
Answered by Prachi Sawant | 06 Sep, 2016, 10:03: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 17 Apr, 2020, 09:22: AM
CBSE 12-science - Chemistry
Asked by adarshkamble130 | 19 Aug, 2019, 12:22: AM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 10:50: PM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 10:47: PM
CBSE 12-science - Chemistry
Asked by Sneha | 16 Dec, 2018, 03:14: PM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:54: AM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:52: AM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 01:00: AM





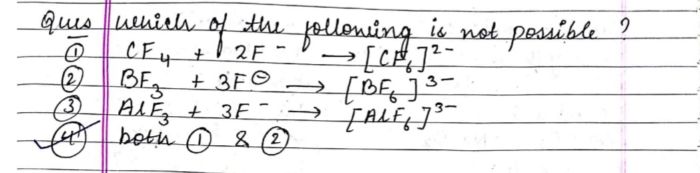
 to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc. 