CBSE Class 12-science Answered
We know that nitrogen can't have +5 oxidation state,so why N2O5 exists?
Asked by ranvirsingh1as | 16 Dec, 2018, 22:50: PM
Actually, you are getting confused in covalency and oxidation state because they both are different things.
Covalency is the combining capacity of an atom.
In the case of nitrogen, it can form three covalent bond and one coordinate bond by its lone pairs which mean Nitrogen has covalency 4.
on the other hand, the oxidation state is the total charge present on an atom in its combined state.
In N2O5, nitrogen is attached to he five oxygen atoms which exert +5 charge on the nitrogen atom.
Hence its covalency is four and oxidation state in N2O5 is +5.
Answered by Ramandeep | 20 Dec, 2018, 11:36: AM
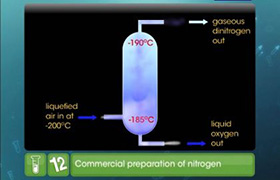
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 19:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 13:06: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 17 Apr, 2020, 09:22: AM
CBSE 12-science - Chemistry
Asked by adarshkamble130 | 19 Aug, 2019, 00:22: AM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 22:50: PM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 22:47: PM
CBSE 12-science - Chemistry
Asked by Sneha | 16 Dec, 2018, 15:14: PM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:54: AM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:52: AM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 01:00: AM



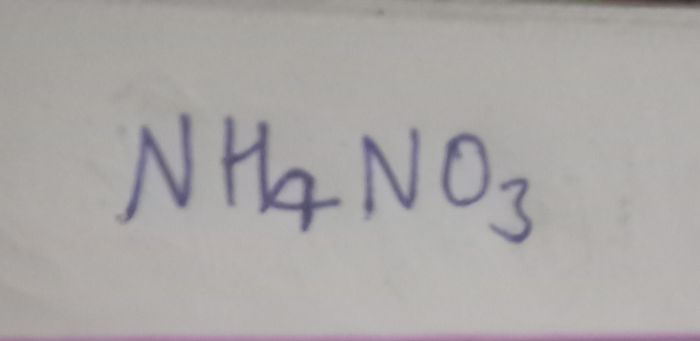
 to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc. 