CBSE Class 12-science Answered
A gas X is formed by heating ammonium nitrite. X can also be obtained by heating barium azide. Mg metal burns in X to form Y. Y reacts with water to form a gas zZwhich turns red litmus blue. Identify X,Y and Z. Write all the reactions involved.
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:54: AM
1. A gas X is formed by heating ammonium nitrite.
X can also be obtained by heating barium azide.
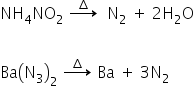
Thermal decomposition of ammonium nitrite and barium azide gives nitrogen gas.
Therefore X = Nitrogen gas
2. Mg metal burns in X to form Y.
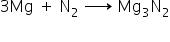
Magnesium reacts with nitrogen to give magnesium nitrite.
Y = Magnesium nitrite.
3. Y reacts with water to form a gas Z which turns red litmus blue.
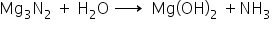
Z is NH3 turns red litmus blue.
Therefore,
X = Nitrogen gas (N2)
Y=Magnesium nitrite. (Mg3N2)
Z=Ammonia (NH3)
Answered by Varsha | 28 Aug, 2018, 15:11: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 19:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 13:06: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 17 Apr, 2020, 09:22: AM
CBSE 12-science - Chemistry
Asked by adarshkamble130 | 19 Aug, 2019, 00:22: AM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 22:50: PM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 22:47: PM
CBSE 12-science - Chemistry
Asked by Sneha | 16 Dec, 2018, 15:14: PM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:54: AM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:52: AM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 01:00: AM




 to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc. 