CBSE Class 12-science - Carbohydrates Videos
Carbohydrates
Carbohydrates
More videos from this chapter
View All- difference between glucose and fructose?
- how many asymmetric carbon atoms are present gulose
- what are carbohydrate
- What are anomers?
- Mass of sucrose C12H22O11 produced by mixing 84 gm of carbon, 12 gm of hydrogen and 56 L. O2 at 1 atm & 273 K according to given reaction, is C(s) + H2(g) + O2(g) → C12H22O11(s)
- Give one example each for reducing and non-reducing sugars.
- Why are carbohydrates generally optically active?
-
An optically active compound having molecular formula C6H12O6 is found in two isomeric forms (A) and (B) in nature. When (A) and (B) are dissolved in water they show the following equilibrium:
(i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
-
Despite having an aldehydic group:(a) Glucose does not give 2, 4-DNP test. What does this indicate?(b) Draw the howorth structure of
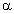 -D-(+)-Glucopyranose.(c) What is the significance of D and (+) here?
-D-(+)-Glucopyranose.(c) What is the significance of D and (+) here?
- Why are carbohydrates generally optically active?