CBSE Class 12-science Answered
An optically active compound having molecular formula C6H12O6 is found in two isomeric forms (A) and (B) in nature. When (A) and (B) are dissolved in water they show the following equilibrium: (i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
(i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
 (i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
(i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
Asked by Topperlearning User | 22 Jun, 2016, 08:00: AM
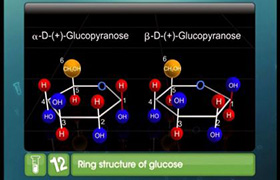
(i) Anomers
(ii) No, they are not enantiomers because stereo isomers related to each other as non superimposable mirror images are enanatiomers. Anomers differ only at C1 configuration.
(iii)
Answered by | 22 Jun, 2016, 10:00: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by praveenk5480 | 31 May, 2021, 11:22: AM
CBSE 12-science - Chemistry
Asked by naikmamata688 | 02 Aug, 2020, 09:41: AM
CBSE 12-science - Chemistry
Asked by shahwajahat1604 | 03 Jun, 2020, 21:10: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 27 Nov, 2019, 12:23: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 19 May, 2018, 00:05: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 07:58: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 07:59: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 08:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 08:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM