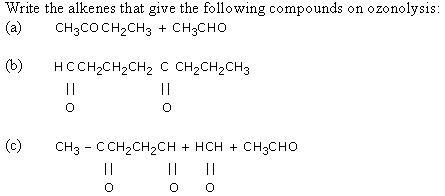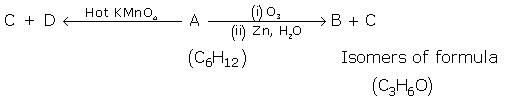CBSE Class 11-science Chemistry Properties of Alkenes
- What happens when sec propyl alcohol react with sulphuric acid at suitable temperature and pressure
- Write two tests to distinguish 1-pentene from n-pentane?
- An alkene on ozonolysis gives 2-butanone and 2-methylpropanal. What products will be obtained when its is treated with hot conc. KMnO4?
- A hydrocarbon containing two double bonds gave on reductive ozonolysis ethanal, glyoxal and prapanone. Predict the structure of the hydrocarbon and give its IUPAC name.
- One mole of a hydrocarbon (A) reacts with one mole of bromine giving a dibromo compound, C5H10Br2. Substance (A) on treatment with cold dilute alkaline KMnO4 solution forms a compound C5H12O2. On ozonolysis (A) gives equimolar quantities of propanone and ethanal. Deduce the structural formula of (A).
- The hydrocarbon 'A' adds on mole of hydrogen in the presence of platinum to form n-hexane. When 'A' is oxidized vigorously with KMnO4, a single carboxylic acid containing three carbon atoms is isolated. Give the structure of 'A' and explain.
- A hydrocarbon 'X' takes up two molecules of hydrogen and is converted into a saturated hydrocarbon. On ozonolysis, 'X' gives a mixture of three carbonyl compounds namely, acetaldehyde, acetone and propan-1, 3-dial. Assign structure to compounds X.
-
-
Give the structure of A and B:
- What is the cause of geometrical isomerism in alkenes?