CBSE Class 10 Answered
Write the chemical equation of the reaction in which the following changes have taken place with an example of each:
(i) Change in colour
(ii) Change in temperature
(iii) Formation of precipitate
Asked by pachchigarkeyur | 08 Mar, 2022, 12:05: PM
(i)Change of colour
Example:
When few pices of Fe are added to the blue copper sulphate solution, the colour of the solution fades
and turns into light green due to the formation of ferrous sulphate.


(ii) Change in Temperature-
The action of dilute sulphuric acid on zinc.
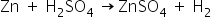
Heat is evolved in this reaction.
(3)Formation of Precipitate-
Example:
When silver nitrate is added to sodium chloride, white insoluble precipitate of silver chloride is formed.
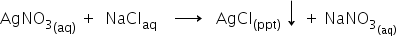 When ferrous sulphate solution is added to the sodium hydroxide, a dirty green precipitate ferrous hydroxide is formed.
When ferrous sulphate solution is added to the sodium hydroxide, a dirty green precipitate ferrous hydroxide is formed.
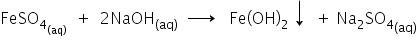
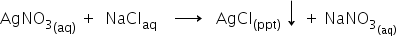
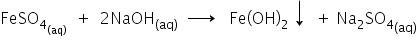
Answered by Ravi | 09 Mar, 2022, 12:03: PM
CBSE 10 - Science
Asked by srinivasansriharinia9 | 01 Mar, 2024, 20:17: PM
CBSE 10 - Science
Asked by muskanprayagraj78 | 26 Feb, 2024, 17:44: PM
CBSE 10 - Science
Asked by technicalbeta720 | 13 Dec, 2023, 07:44: AM
CBSE 10 - Science
Asked by latayadav23 | 14 Oct, 2023, 12:21: PM
CBSE 10 - Science
Asked by labheshvaidya | 28 Jul, 2022, 16:31: PM
CBSE 10 - Science
Asked by deshmukhparth2007 | 08 Apr, 2022, 23:39: PM
CBSE 10 - Science
Asked by pachchigarkeyur | 08 Mar, 2022, 12:05: PM
CBSE 10 - Science
Asked by pachchigarkeyur | 08 Mar, 2022, 12:03: PM

