CBSE Class 10 Answered
Write a short note on electrolytic refining with example. Give diagram.
Asked by Topperlearning User | 24 Jul, 2017, 14:12: PM
Electrolytic refining:- Many metals, such as copper, zinc, tin, nickel, silver, gold etc. are refined by electrolysis. In this process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. The solution of the metal salt is used as an electrolyte. When current passes through electrolyte, the pure metal from the anode dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the cathode. The insoluble impurities settle down at the bottom of the anode and are known as anode mud whereas the soluble impurities go into the solution.
For example: In electrolytic refining of copper, the electrolyte is a solution of acidified copper sulphate. The anode is impure copper, whereas the cathode is a strip of pure copper. On passing electric current, pure copper is deposited on the cathode.
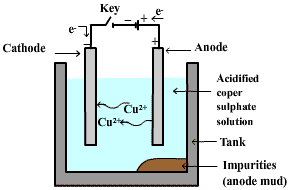
Answered by | 24 Jul, 2017, 16:12: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by 83dimplejain29 | 01 Sep, 2022, 15:04: PM
CBSE 10 - Chemistry
Asked by dibyendumukherjee451 | 31 May, 2022, 11:51: AM
CBSE 10 - Chemistry
Asked by advssdrall | 28 Oct, 2020, 17:05: PM
CBSE 10 - Chemistry
Asked by dafk04.dp | 30 Sep, 2020, 12:34: PM
CBSE 10 - Chemistry
Asked by morev1725 | 06 Sep, 2020, 17:57: PM
CBSE 10 - Chemistry
Asked by ashishpatidar1009993 | 25 May, 2020, 16:29: PM
CBSE 10 - Chemistry
Asked by rmchougule5 | 17 Apr, 2020, 16:17: PM
CBSE 10 - Chemistry
Asked by subbukum | 18 Feb, 2020, 17:38: PM
CBSE 10 - Chemistry
Asked by akashkumardobhi273 | 01 Feb, 2020, 11:05: AM
CBSE 10 - Chemistry
Asked by saradamahapatra9999 | 06 Dec, 2019, 10:06: AM







