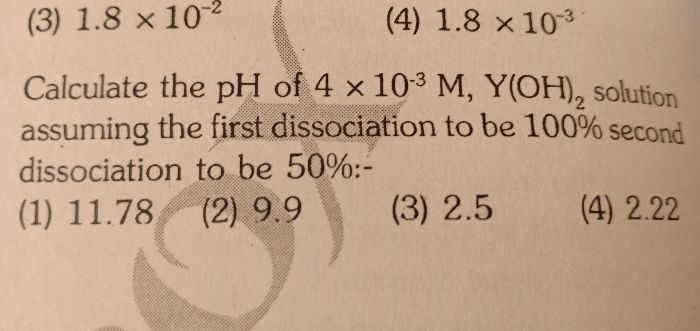CBSE Class 11-science Answered
why would the basic strength of NH4OH decrease on addition of NH4Cl ?
Asked by astutijoshi | 15 Sep, 2019, 03:09: PM
NH4OH is a weak base it does not ionizes completely, while NH4Cl is a strong electrolyte, It decomposes completely. Due to the presence of NH4+ in NH4Cl, It suppresses the ionisation of weak base NH4OH to decrease the OH- ion concentration so that higher group cations will not get precipitated. So basic strength of NH4OH decreases. This effect is known as the common ion effect.
Answered by Ravi | 16 Sep, 2019, 12:20: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:50: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:44: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 14 Apr, 2020, 02:42: PM
CBSE 11-science - Chemistry
Asked by SanskarAgarwal86 | 29 Feb, 2020, 04:36: AM