CBSE Class 12-science Answered
Why nucleophilic substitution reaction do not take place in aryl halide and alkyl halide
Asked by priadkonkar | 21 Jan, 2019, 20:52: PM
There is an interaction between one of the lone pairs on the halogen atom and the π electrons of the ring, and this strengthens the bond.
So it is difficult to break the bond.
The electron-withdrawing polar effect of the ring double bonds destabilises an aryl cation.
They require very high energy for the formation of carbocation intermediate -aryl cation.
Thus making SN1 reactions impossible.
Answered by Varsha | 21 Jan, 2019, 22:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 13:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 17:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 16:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 22:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 13:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 14:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 20:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 14:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 14:34: PM




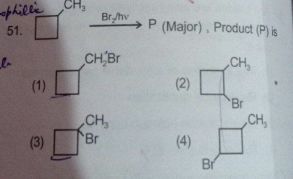
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-