JEE Class main Answered
why it doesn't undergo ring expansion
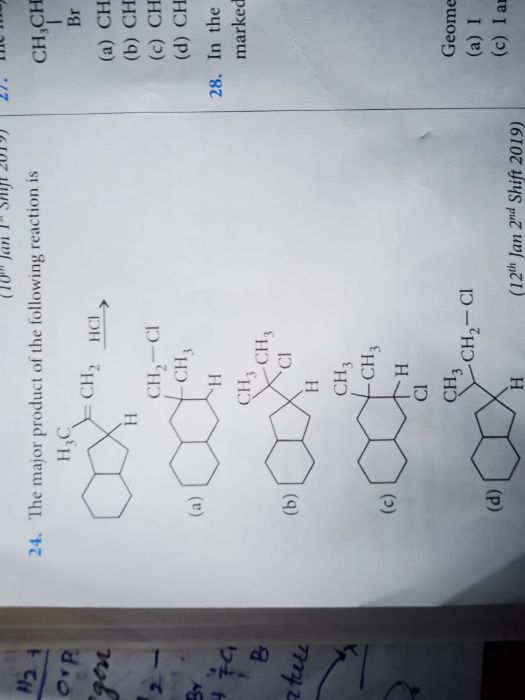
Asked by sirikondagangothri186 | 06 Apr, 2022, 09:22: AM
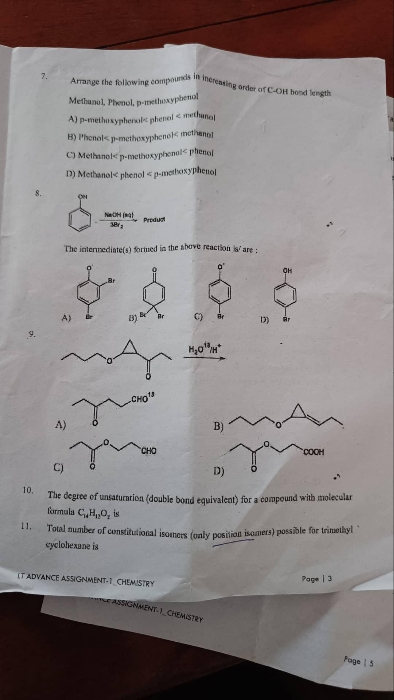
In most of the cases ring expansion occurs when the there is need to stabilise a carbocation. But in the give reaction the carbocation formed during reaction is tertiary which is most stabilised form of carbocation hence there are only 1% chances of ring expansion in this case and we get option B as a major product with 99%.
For more details please click here
Answered by Ramandeep | 06 Apr, 2022, 11:16: AM
JEE main - Chemistry
Asked by sirikondagangothri186 | 06 Apr, 2022, 09:22: AM
JEE main - Chemistry
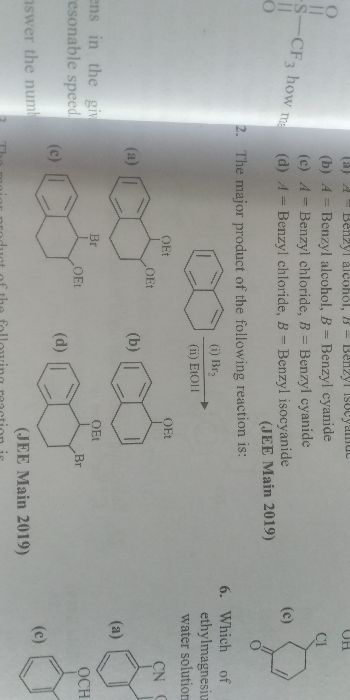
Asked by b2112342 | 31 Dec, 2021, 14:35: PM
JEE main - Chemistry
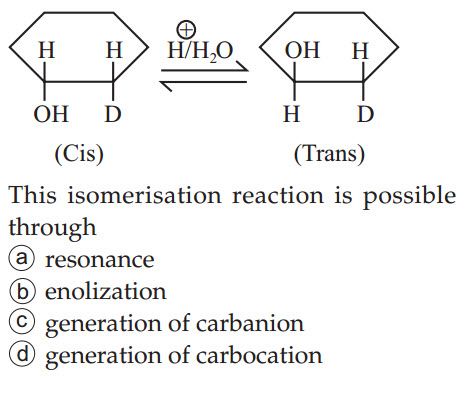
Asked by ankur.recjpr | 21 Oct, 2019, 00:31: AM
JEE main - Chemistry
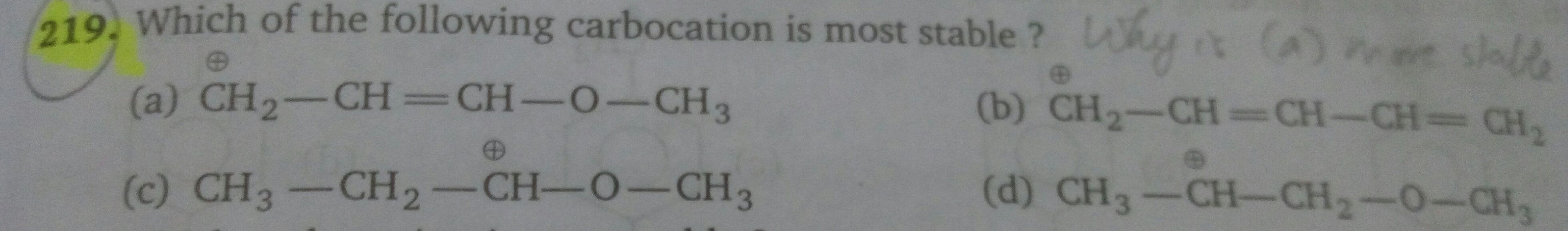
Asked by sumayiah2000 | 01 May, 2019, 13:22: PM
JEE main - Chemistry
Asked by sumayiah2000 | 30 Apr, 2019, 20:32: PM