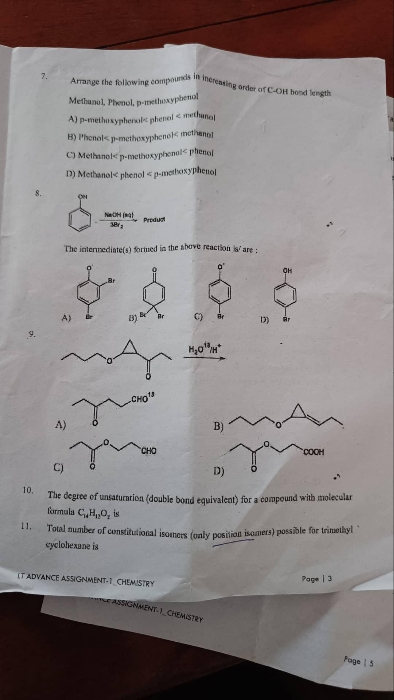
JEE Class main Answered
Why is option (a) said to be the most stable and not option (b) ?
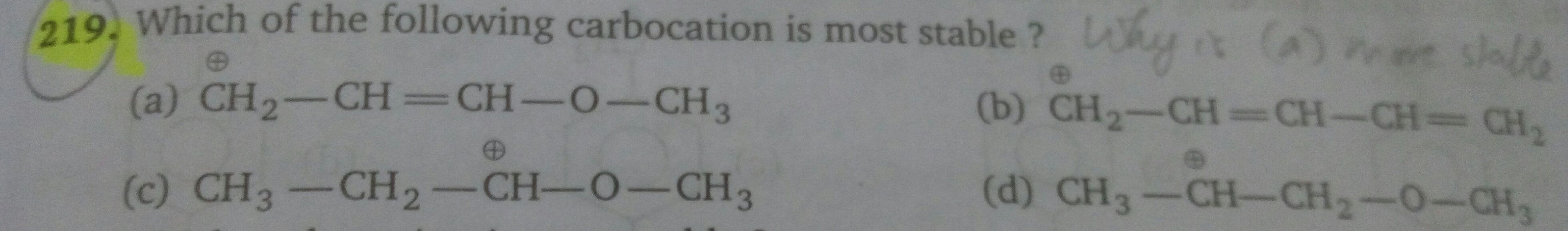
Asked by sumayiah2000 | 30 Apr, 2019, 20:32: PM
Here, In this case, you can remember the following key points:
Given compound is always more stable than its resonating structures (Charged species are less stable than uncharged)
No. of covalent bonds increases, stability increases.
Unlike charge separation (distance between opposite charges) decreases stability increases while like (distance between same charges) charges increases stability increases.
Negative charged is least stable at the less electronegative atom while it is more stable at a more electronegative atom.
In the given question,
Option D: In this compound, the carbocation is secondary hence it is least stable than rest.
Option C: A carbocation is attached to -OCH3 group which exerts only +I effect but still it is not more stable than the rest.
option B: Threre is allylic carbocation is present, which can be satbilised by the +M effect,
Option A: In this compound the carbocation is in conjugation with -OCH3 which exerts +I and +M effect hence this carbocation is most stable.
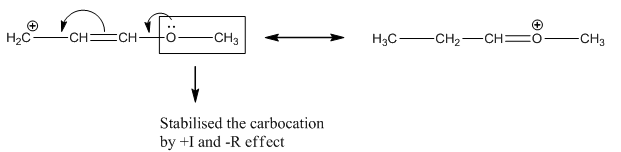
Answered by Ramandeep | 02 May, 2019, 11:39: AM
JEE main - Chemistry
Asked by sirikondagangothri186 | 06 Apr, 2022, 09:22: AM
JEE main - Chemistry
Asked by b2112342 | 31 Dec, 2021, 14:35: PM
JEE main - Chemistry
Asked by ankur.recjpr | 21 Oct, 2019, 00:31: AM
JEE main - Chemistry
Asked by sumayiah2000 | 01 May, 2019, 13:22: PM
JEE main - Chemistry
Asked by sumayiah2000 | 30 Apr, 2019, 20:32: PM